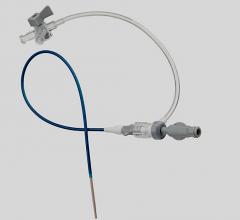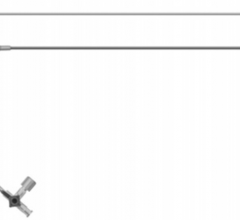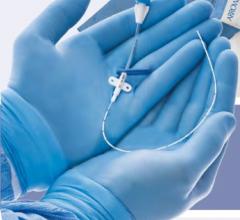
December 21, 2010 – A new introducer kit is now available in the United States. The VSI StraitSet Kit, from Vascular Solutions, contains a triaxial introducer comprising a 6 French, a 20 cm sheath, inner dilator and metal stiffening cannula.
The sheath features an embedded radiopaque marker band at the distal tip and is available with either a hydrophilic coating or a silicone wipe. Included with the introducer in the kit is a 0.018-inch by 60-cm nitinol mandrel guidewire with a radiopaque platinum coil tip, and a 21 gage needle in either a 7-cm echogenic or a 15-cm trocar version.
Interventionalists can choose between the VSI StraitSet, or the existing GrebSet microintroducer kit, which adds the feature of an angled tip to the introducer for steering the guidewire during placement. The sterile, single-use VSI StraitSet and GrebSet are available in the United States exclusively through Vascular Solutions.
For more information: visit www.vascularsolutions.com

 June 10, 2020
June 10, 2020