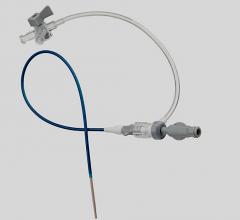
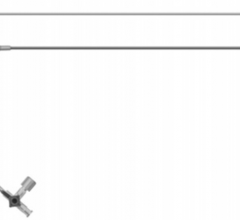
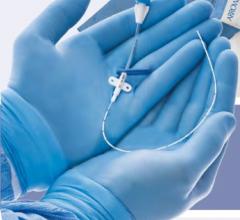
September 11, 2009 – Terumo Interventional Systems yesterday announced the expansion of its Pinnacle Destination Guiding Sheaths with an 8 French size to extend the range of interventional procedures that can be performed, including atherectomy and stent graft procedures.
The Pinnacle Destination Guiding Sheath line supports virtually all applicable diagnostic and interventional procedures, the company said. The brand now offers a range of French sizes (from 5-8), lengths and tip shapes as well as two different valve types.
The company said the guiding sheath design includes a unique atraumatic tip, which minimizes the potential for vessel damage, allows smooth transition from guide wire to dilator and dilator to sheath, helping to ensure easy penetration without force. The sheath has a hydrophilic coating on a nylon outer layer, with a PTFE inner layer and stainless steel coil.
For more information: www.terumois.com

 June 10, 2020
June 10, 2020