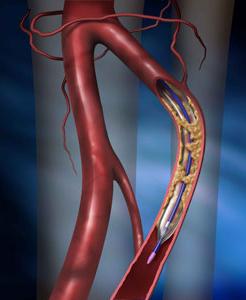
May 19, 2010 – A high-performance balloon dilatation catheter designed for use in peripheral angioplasty procedures below the knee was released today in both the United States and Europe. Boston Scientific said the Sterling SL Percutaneous Transluminal Angioplasty (PTA) Balloon Dilatation Catheter was developed specifically to address physicians’ needs in treating below-the-knee arteries.
The balloon has a low tip profile, offers easy deliverability and rapid deflation time. It expands the company’s line of low-profile peripheral angioplasty balloon catheters, which includes the Sterling and Sterling ES products.
“The introduction of the Sterling SL Balloon Catheter will provide the length options I need to address below-the-knee procedures for patients with peripheral artery disease,” said Kenneth Kollmeyer, M.D., FACS, DFW Vascular Group and chief of vascular surgery, Methodist Dallas Medical Center. “This product offers the same excellent performance I have come to expect from the Sterling family of balloon catheters.”
For more information: www.bostonscientific.com


 June 13, 2024
June 13, 2024 









