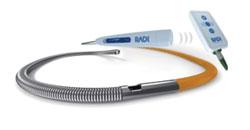
October 20, 2008 – Radi Medical Systems displayed its new wireless PressureWire Aeris to measure fractional flow reserve (FFR) last week during the Transcatheter Cardiovascular Therapeutics (TCT) 2008 in Washington, D.C.
FFR helps guide PCI procedures for improved patient outcomes and lowered cost. FFR is an index for functional severity of coronary stenosis based on blood pressure flowing around a lesion. The technology helps identify which lesion or lesions are responsible for a patient’s ischemia, enabling a cardiologist to make more informed-choices as to which areas to treat, instead of relying on images of the vessels alone. The idea is that not all lesions need to be stented, and fewer stents mean lower costs and shorter procedure times. Radi said many lesions can be treated with medication alone instead of stents or angioplasty, which may leave hardware behind or damage vessels, possibly creating complications later.
Radi said its recently approved PressureWire Aeris is the first wireless device introduced for the cath lab to help reduce wire obstructions.
During TCT results of the latest landmark study to demonstrate the clinical value of FFR, FAME, were presented. The one-year follow-up of the FAME study (FFR vs. angiography for multi-vessel evaluation) confirms the beneficial clinical and economic impact of FFR when used routinely to guide treatments of multi-vessel disease. FAME randomized 1,005 patients diagnosed with multi-vessel coronary artery disease at 20 European and U.S. centers to either angiography-guided PCI or FFR-guided PCI all using current DES regimens. FAME demonstrated that FFR-guidance in routine multi-vessel PCI resulted in clinically-superior outcomes and reduced the composite of death, documented myocardial infarction, and repeat revascularization by 30 percent at one year. The study also demonstrated that adhering to an FFR-guided regimen for multi-vessel disease is cost beneficial to the hospital and payers by reducing procedural costs ($5,332 vs. $6,007), lowering average length of hospitalization (3.4 days vs. 3.7 days), and reducing the number of drug eluting-stents necessary per patient (1.9 vs. 2.7). The data also showed that the implementation of FFR did not add time to the multi-vessel PCI procedure.
GE Healthcare and Radi announced Oct. 13 at TCT a development, marketing and sales initiative to integrate Aeris Pressurewire FFR technology into GE’s Mac-Lab IT hemodynamic recording system. GE said the technology will be seamlessly integrating FFR into the cathlab workflow and with all procedural results stored into existing data archives. The initiative will also provide an upgrade path for GE Mac-Lab IT customers to add this functionality to their existing systems.
“Traditionally, FFR assessment required additional capital equipment and increased procedure time due to setup operations.” said Dr. Jasvindar Singh, associate professor of Medicine at Washington University School of Medicine, Barnes Jewish Hospital, St. Louis, MO. “With FFR fully integrated into our GE Mac-Lab IT, I’ll be able to make the best treatment options immediately available to my patients using equipment we already have, without any installation of additional instrumentation, screens or controls. Additionally, the wireless technology of PressureWire Aeris removes cables crossing the sterile field barrier, making the entire procedure faster and easier.”
For more information, visit www.radi.se, www.gehealthcare.com


 October 28, 2025
October 28, 2025 









