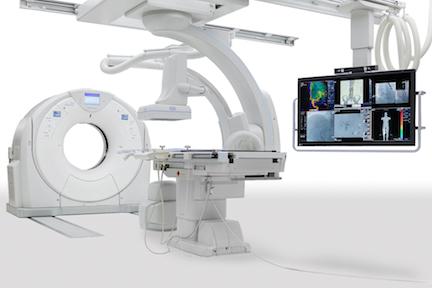
December 14, 2015 — The industry’s first seamless integration of interventional radiology (IR) and CT technology into one solution, Toshiba America Medical Systems’ Infinix 4DCT allows clinicians to plan, treat and verify in a single clinical setting. Toshiba showcased the Infinix 4DCT’s merging of the Infinix Elite angiography system and Aquilion One Vision edition CT system at the 2015 annual meeting of the Radiological Society of North America (RSNA).
The Infinix Elite provides precision and flexibility during intervention, while the Aquilion One Vision is capable of capturing an entire organ in one rotation with 640 slices and 16 cm of anatomical coverage, producing image quality that far exceeds CT-like imaging of the interventional lab. The combination of the interventional lab and CT eliminates the need to transfer patients between departments and allows clinicians to decrease procedure time and maintain patient safety. Toshiba’s third-generation iterative dose reconstruction software, AIDR 3D (Adaptive Iterative Dose Reduction 3D) also offers simplified CT dose reduction that produces the image quality needed at a low dose.
For more information: www.medical.toshiba.com


 October 24, 2025
October 24, 2025 









