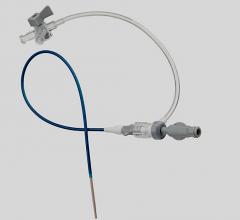
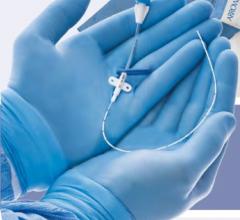
November 3, 2009 – Vascular Solutions Inc. recently launched the Guardian II Hemostasis Valve. Unlike conventional rotating hemostasis valves, the Guardian II has an ergonomic click-open and click-close design to provide protection during interventions and separation of multiple guide wires and other devices used in the catheterization procedure.
The company said a new and improved design of the original Guardian, the Guardian II has a shorter overall length, clear rotating components, and intuitive symbols added to the device.
Manufactured by Zerusa Limited of Galway, Ireland, the hemostasis valve uses proprietary seal technology to provide independent movement of, and a complete seal around, each device. With its ergonomic click-open and click-close design, the device fits comfortably in the hand and allows for easy one-hand operation. The Guardian II has an 8 Fr. compatible lumen to allow delivery of multiple therapeutic devices and a secure device lock to prevent unwanted wire or device movement.
For more information: www.vascularsolutions.com

 June 10, 2020
June 10, 2020