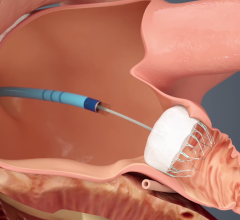
March 4, 2016 — Boston Scientific has received U.S.
W. L. Gore & Associates (Gore) announced the availability of a new 7.5 cm length of the Gore…
Vital Connect debuted the VitalPatch biosensor at the 2016 Healthcare Information and Management…
Avinger Inc. announced the company has received 510(k) clearance from the U.S. Food and Drug…
Philips announced four leading U.S. health systems have signed multi-year telehealth deals to…
Philips announced that it will introduce a next-generation monitoring solution for at-risk…
February 29, 2016 — APN Health LLC announced it received U.S.
February 23, 2016 — WITS(MD), a provider of medical image workflow solutions, announced a…
Doctablet is an online educational video resource that uses vibrant animation and simple…
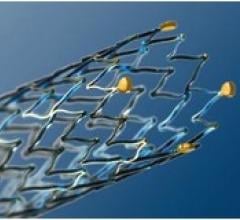
Nipro North America announced the launch of the AquaLiner II second-generation 0.035-inch,…
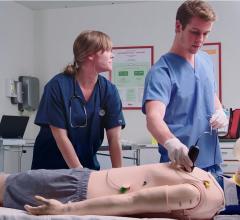
Laerdal Medical, provider of simulation solutions for healthcare, and SonoSim Inc., provider of…
Biotronik announced a partnership with Maquet Medical Systems USA to distribute Biotronik…
Royal Philips announced U.S. Food and Drug Administration (FDA) 510(k) clearance for the…
February 9, 2016 — Boston Scientific Corp.
St. Jude Medical Inc. announced the launch of the company’s Optis Mobile System in Japan and…
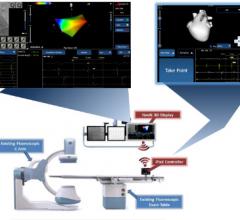
February 4, 2016 — Stereotaxis and Philips have signed an addendum pursuant to their existing…
On February 1, the first day of American Heart Month, the American Heart Association (AHA)…
Boston Scientific Corp. and Accenture have developed a cloud-based, data-driven digital health…
Conavi Medical Inc. (formerly Colibri Technologies Inc.) has received U.S. Food and Drug…
January 26, 2016 — Fujifilm SonoSite Inc. announced CE mark and U.S.

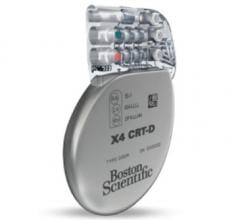
 March 04, 2016
March 04, 2016