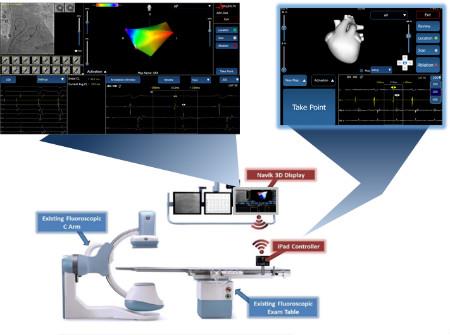
February 29, 2016 — APN Health LLC announced it received U.S. Food and Drug Administration (FDA) clearance to market Navik 3D, an advanced cardiac mapping system that reduces the cost and complexity of electrophysiology procedures. Navik 3D gives electrophysiologist/cardiologists real-time 3-D catheter location in the heart from the 2-D fluoroscopic images; the system then correlates them with the electrical activation of the heart to create 3-D maps of the cardiac chamber of interest in abnormal heart rhythms such as atrial fibrillation.
Navik 3D is the first cardiac mapping system that does not require specialized equipment, according to the company. Instead it uses the patient monitoring and fluoroscopic imaging systems already present in electrophysiology labs. The Navik 3D clearance was supported by extensive data, demonstrating Navik 3D's ability to accurately identify catheter locations in the heart and create 3-D maps of the cardiac chamber of interest.
To assist in the procedure workflow, the system includes a workstation and iPad. It displays real-time 3-D cardiac maps in a number of different formats, including anatomical maps, cardiac electrical activation maps and cardiac voltage maps.
For more information: www.apnhealth.com


 January 29, 2026
January 29, 2026 









