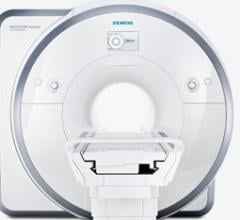
July 31, 2012 — Siemens Healthcare announced that the Magnetom Spectra 3.0T magnetic resonance…
July 30, 2012 — Emerge Clinical Solutions, a creator of clinical decision support software,…
July 27, 2012 — Cook Medical announced general availability of the Aprima Access nonvascular…
July 27, 2012 — Healthcare imaging specialist Barco announces the release of a new version of…
July 26, 2012 — Transgenomic Inc. has announced that National Government Services, the…
July 24, 2012 — The importance of utilizing…
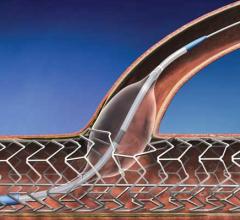
July 24, 2012 — GE Healthcare announced U.S. Food and Drug Administration (FDA) 510(k) clearance…
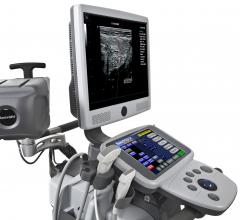
July 23, 2012 — Siemens Healthcare expands its ultrasound portfolio with the portable Acuson…
July 20, 2012 — Crux Biomedical announced it has received U.S. Food and Drug Administration (FDA…
July 20, 2012 — Sony Electronics is announcing the world's first…
July 17, 2012 — Panasonic announced a new 3-D medical-grade 32-inch class monitor, the EJ-MDA32U…
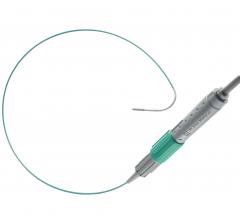
July 16, 2012 — TriReme Medical Inc. (TMI) announced today that it has received U.S. Food and…
July 13, 2012 — Angioslide Ltd., a provider of embolic capture angioplasty solutions, today…
July 11, 2012 — Ultrasonix Medical Corporation has received approval from the U.S. Food and Drug…
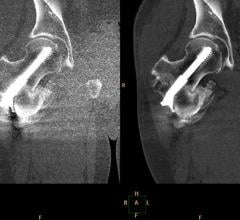
July 11, 2012 — St. Jude Medical received 510(k) clearance from the U.S. Food and Drug…
July 9, 2012 — r4 Vascular Inc. announced clearance from the U.S. Food and Drug Administration (…
June 22, 2012 — Siemens Healthcare has announced that the U.S. Food and Drug Administration (FDA…
June 22, 2012 — Philips introduced the iDose4 Premium Package, which includes two…
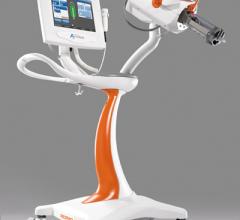
June 22, 2012 — Bayer HealthCare widens its portfolio of interventional solutions by introducing…
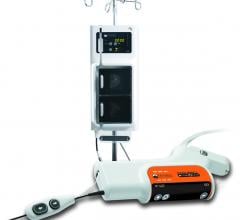
June 22, 2012 — Bayer HealthCare’s Radiology and Interventional unit has launched the new Mark 7…

 July 31, 2012
July 31, 2012