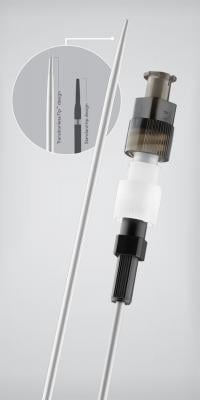
July 27, 2012 — Cook Medical announced general availability of the Aprima Access nonvascular introducer set, the first product in the Aprima drainage portfolio. It is a nonvascular access set used for single-puncture percutaneous access to facilitate placement of a working wire guide for interventional radiology (IR) procedures.
Key to the performance of the Aprima Access product platform is the transition-less design of the sheath and the introducer. The Transitionless-Tip is designed to help interventional radiologists streamline access and reduce the risk of patient trauma by substantially reducing any major resistance that could make insertion difficult.
“Cook was among the first to create drainage products specifically engineered to meet the needs of interventional radiologists,” said Dan Sirota, vice president and global leader of Cook Medical’s Interventional Radiology and Critical Care divisions. “Decades of collaborating with physicians to provide these solutions have given Cook a deep understanding of patient and physician needs in this market. This knowledge has helped Cook continue to advance the technology needed for every drainage procedure.”
The set's design is highly radiopaque along the entire shaft, including the distal tip, to provide visibility under fluoroscopy after placement. The set also includes a hydrophilic-coated coaxial introducer, which works with the Transitionless-Tip to ease placement for drainage access. Physicians purchasing the complete set also have a choice of two EchoTip needles designed for optimal ultrasound visibility.
Designed to accommodate physician preference, the complete Aprima Access set options include a choice of two types of EchoTip echogenic access needles, two types of wire guides and the hydrophilic-coated coaxial introducer. The Aprima Access Set is currently available to customers in the United States and Canada.
For more information: www.cookmedical.com

 November 13, 2025
November 13, 2025