Omron Healthcare Inc. and physIQ Inc. announced a collaboration to integrate Omron’s HeartGuide wearable blood pressure monitoring device into the pinpointIQ platform to monitor at-risk patients in an outpatient setting. The strategic collaboration aims to generate actionable clinical information for at-risk patients in an outpatient environment by leveraging the complementary technologies of each company.
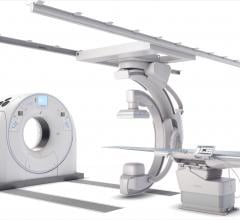
Canon Medical Systems USA Inc. recently introduced a new angiography configuration featuring its Alphenix Sky + C-arm and Hybrid Catheterization Tilt/Cradle Table for interventional procedures with its Aquilion One/Genesis Edition computed tomography (CT) system. The new pairing, called the Alphenix 4-D CT, allows clinicians to efficiently plan, treat and verify in a single clinical setting. The flexible hybrid system enables streamlined workflow and extensive range of patient access and coverage.
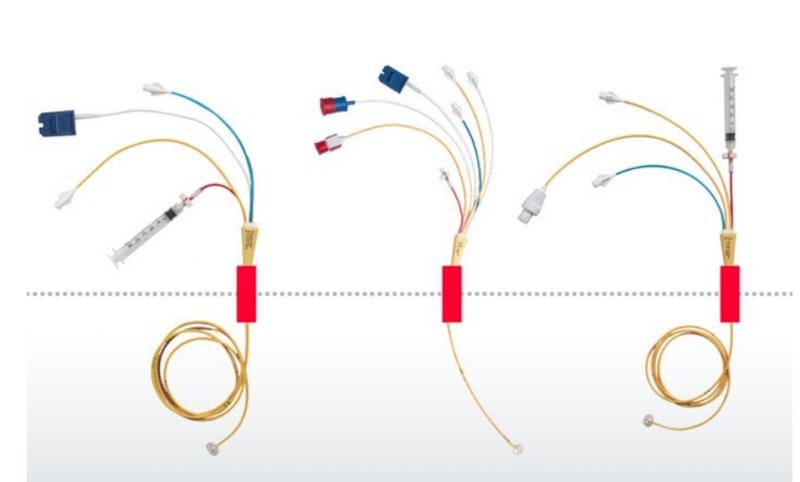
Edwards Lifesciences is recalling its 131F7, 131F7J, 131F7P, 131VF7P, 151F7 Swan-Ganz Thermodilution Catheters ...
Cardiac PET/CT represents a major advancement in cardiovascular diagnostics, offering significant clinical and ...
February 5, 2019 – The American Society of Echocardiography (ASE) released a new document that provides a comprehensive ...
Stroke survivors have better quality of life three months after their stroke if the clot that caused the stroke was mechanically removed even hours beyond the ideal treatment window compared to those treated with drugs alone. This preliminary research will be presented, Feb. 6-8 in Honolulu at the American Stroke Association’s International Stroke Conference 2019.
The U.S. Food and Drug Administration (FDA) sent a letter to cardiologists this week to explain its evaluation of high ...
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
In 2018, capital investment in companies developing artificial intelligence (AI)-enabled medical image analysis solutions was almost $580 million. This was more than double the investment into such companies in 2017, according to market research firm Signify Research.
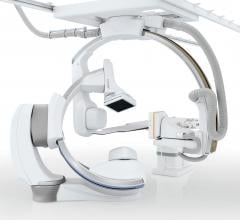
Canon Medical Systems USA recently introduced its next generation of interventional systems – the Alphenix platform. The new flagship platform of systems incorporates all-new features that enable clinicians to deliver images with clarity and precision without compromising workflow and while prioritizing low dose.
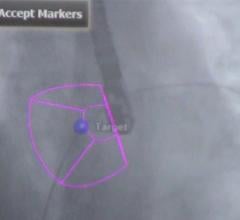
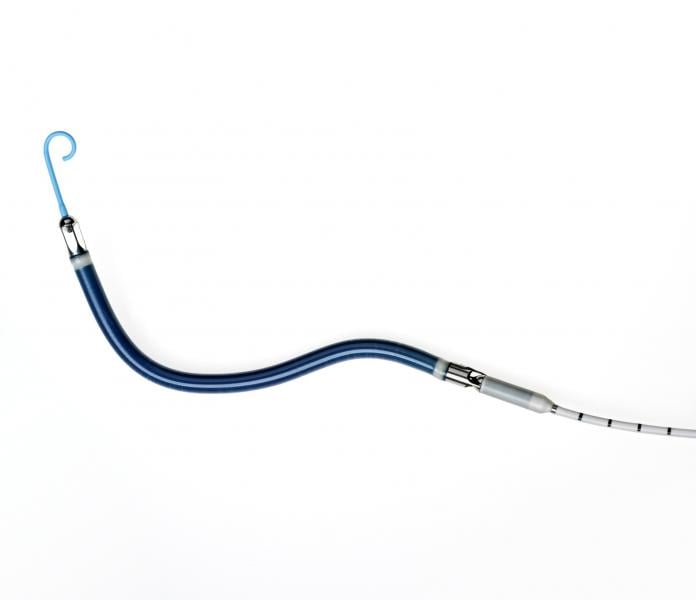
Biosense Webster has enrolled and treated the first patient in its U.S. Investigational Device Exemption (IDE) study evaluating the company’s QDot Micro radiofrequency (RF) ablation catheter in the treatment of symptomatic drug-refractory paroxysmal atrial fibrillation (AF). The first AF patient was treated at NYU Langone Health’s Heart Rhythm Center in New York City, one of up to 30 centers participating in the study that will enroll up to 185 patients throughout the U.S.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

February 4, 2019 — Stryker announced Feb. 1, the company is launching a voluntary field action on specific units of the ...
Paragonix Technologies Inc. recently received clearance from the U.S. Food and Drug Administration (FDA) for a design enhancement allowing for the use of the Paragonix SherpaPak Cardiac Transport System (CTS) with small and pediatric donor hearts. Starting in Q1 2019, the system will now be shipped with heart connectors covering most aortic diameters, permitting the anchoring of various size hearts including small pediatric hearts to its proprietary suspension system for improved donor heart transport.
A team of Rutgers scientists have taken an important step toward the goal of making diseased hearts heal themselves – a new model that would reduce the need for bypass surgery, heart transplants or artificial pumping devices.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...

February 1, 2019 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
Merit Medical Systems Inc. announced that the PreludeSync Distal Compression Device is now available in the United States, European, Middle Eastern, African and Asia-Pacific markets. The device is the first specifically designed to achieve hemostasis of the radial artery during procedures where access is gained via the distal radial artery.
Cardiovascular Systems Inc. announced that the first patients in the United States were treated using the OrbusNeich Teleport Microcatheter (Teleport), which recently received U.S. Food and Drug Administration (FDA) 510(k) clearance.


 February 06, 2019
February 06, 2019