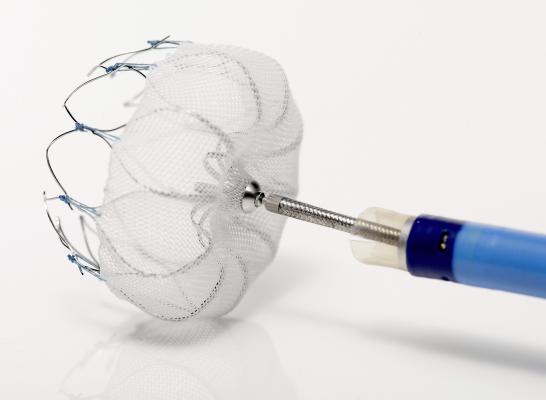
Boston Scientific's Watchman LAA occluder device is designed to prevent stroke in patients atrial fibrillation without the need for warfarin therapy.
November 8, 2013 – According to Millennium Research Group (MRG), the anticipated Food and Drug Administration (FDA) approval of Boston Scientific's Watchman device will drive rapid growth in the market for global left arterial appendage (LAA) closure devices.
Although a number of epicardial LAA closure devices are available worldwide, the Watchman device will represent the first FDA-approved endocardial LAA device when it enters the market in the latter half of 2013. Approval is more difficult to obtain for endocardial devices because they are placed directly inside the LAA opening of the heart, whereas epicardial devices function from outside of the heart.
"Endocardial LAA closure devices have huge global market potential," said MRG Senior Analyst Sean Messenger. "These devices will expand the eligible patient pool for LAA closure because they will be approved for stand-alone use in the US, as they are in Europe. By contrast, the majority of epicardial devices are only approved for concomitant use when patients are undergoing open cardiac surgery for another condition. Physicians are also familiar with endocardial devices given that interventional cardiologists have been using very similar devices for many years."
Market growth will be particularly strong in the United States, where clinical trial results from the PROTECT-AF and PREVAIL studies will support device approval and encourage physician adoption of the Watchman device. FDA approval is also highly regarded in Europe, and as a result, the Watchman device will see strong uptake in this market as well. The device will not enter the Japanese market until it has been well-established in the United States and Europe, although market growth is expected to be strong once endocardial LAA devices enter this space.
St. Jude Medical, the current global leader of the heart defect closure device market, is also expected to eventually enter the endocardial LAA closure device segment; the company recently began a U.S.-based trial for a device in this space. This is not expected to occur for some time, however, and Boston Scientific will enjoy a first-to-market advantage, monopolizing this market for a number of years.
Millennium Research Group's Global Markets for Heart Defect Closure Devices 2013 report includes unit, average selling price and revenue information, along with market drivers and limiters and a competitive landscape for congenital heart defect, patent foramen ovale (PFO) and LAA closure devices in the United States, France, Germany, Italy, the United Kingdom and Japan.
For more information: www.MRG.net


 July 08, 2024
July 08, 2024 









