There are several new interventional and minimally invasive surgical heart failure (HF) devices in development or in trials that might offer new ways to boost patient volume in the coming years. There is a lot of potential economic opportunity in new HF therapies, since HF represents the single-largest cause of hospitalizations in many countries and it is the largest expense in the U.S. Medicare budget.
These minimally invasive technologies come in many varieties to address different aspects of HF, including devices to change the structure of an enlarged heart, counter-pulsation therapy, intra-cardiac pressure relief using and intra-arterial shunt, and pacemakers to control the sympathetic nervous system’s involvement. Here are a few of these technologies to watch.
Implantable Heart Failure Monitor
One of the biggest advances in HF technology is the St. Jude Medical
CardioMEMS implantable heart failure monitor. It was cleared by the U.S. Food and Drug Administration (FDA) in May 2014. The device is inserted into the pulmonary artery (PA) via a transcatheter procedure and accurately monitors pressure to detect fluid retention due to worsening congestive heart failure symptoms. It can detect changes prior to a patient needing hospitalization, when they can be treated with medication, which is hoped to reduce the massive numbers of heart failure readmissions. The system allows clinicians to stabilize PA pressures by proactively managing medications and other treatment options while also providing an early indication of worsening HF.
The Centers for Medicare and Medicaid Services (CMS) have targeted HF to reduce the staggering costs of treating patients in an HF crisis, rather than less expensive outpatient treatments. CMS is targeting these re-admissions with penalties to force hospitals to better manage these patients. In 2014, HF re-admissions cost a hospital 2 percent of its reimbursement rate. In 2015, this will rise to 3 percent, which will likely cost some hospitals millions of dollars a year.
Last August, CMS approved a new technology add-on payment (NTAP) for the CardioMEMS. The NTAP program, which recognizes new technologies that provide substantial clinical improvement over already available therapies, is designed to support timely access to innovative technologies for Medicare beneficiaries. As of October 2014, CMS started reimbursing hospitals an incremental amount in addition to the Medicare Severity Diagnosis Related Group (MS-DRG) payment.
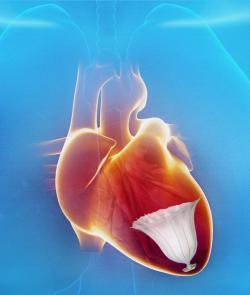
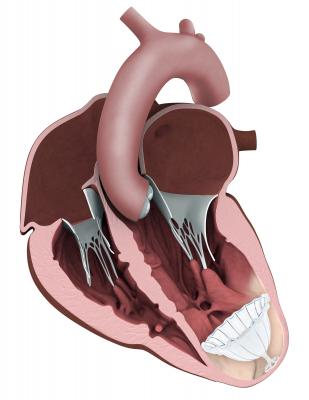
A Parachute to Save Failing Hearts
Following a heart attack, many HF patients suffer from enlargement of the left ventricle (LV) as it attempts to compensate for the loss of myocardium. This diminishes the amount of blood the heart can pump. Treatment options are currently limited to implantable cardioverter-defibrillator (ICD) and cardiac resynchronization therapy devices (CRT-D). The first minimally invasive catheter-based device to restore normal LV geometry and function is the
CardioKinetix Parachute Ventricular Partitioning Device. It received European CE mark in 2011 and is currently being tested in the PARACHUTE IV U.S. clinical trial. Data from the U.S. trial will be used for an FDA pre-market approval (PMA) submission.
The Parachute implant partitions the damaged muscle, isolating the non-functional muscle segment from the functional segment to decrease the overall volume of the LV. The procedure is performed in the cath lab under limited sedation.
Twelve-month clinical data from the PARACHUTE III study of 100 post-market European patients were presented at TCT 2014. It had a high procedural success rate of 97 percent. The primary safety endpoint yielded a low 7 percent rate of device- or procedure-related major adverse cardiac and cerebrovascular events (MACCE). Data show reduced left ventricle volumes resulting in significant improvements in systolic function and a significant reduction in left atrial volume reflected improved diastolic function. New York Heart Association (NYHA) heart failure functional class improved or was maintained in 80 percent of patients. Rates of death and the combined endpoint of death and repeat hospitalization for heart failure were 9.5 percent and 26 percent, respectively.
Edwards Lifesciences Corp. led investors in a $50 million round of financing for CardioKinetix at the end of 2014. The funds provide CardioKinetix with the capital needed to complete the PARACHUTE IV trial. CardioKinetix also entered into an agreement that provides Edwards Lifesciences with the right to acquire the company based on future regulatory milestones.
Intra-arterial Shunts
Another transcatheter technology being developed for heart failure is the intra-arterial shunt device (IASD) by DC Devices. It is a permanent heart implant to treat diastolic HF with preserved ejection fraction (HFpEF) by relieving blood pressure on the left side of the heart and redistributing it to the right side. The device is placed as a permanent implant in the atrial septum. It takes about 15 minutes to implant the device in the atrial septum and it is hoped to help reduce readmission rates and eliminate surgical costs.
Last July, investors pumped $34 million into DC Devices so it could complete its clinical evaluation of the device.
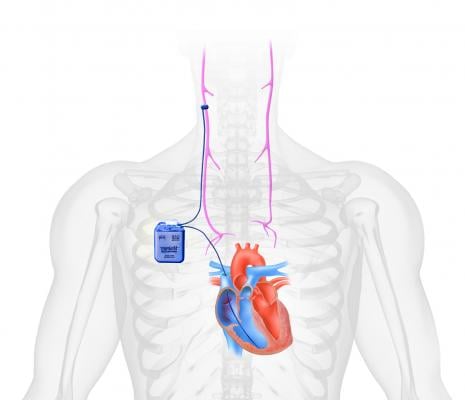
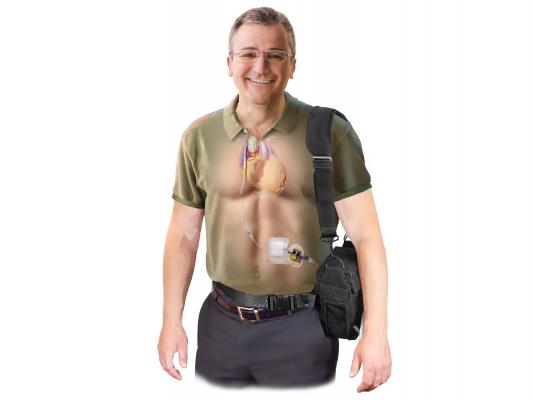
Implantable Counter-pulsation Therapy
The
Sunshine Heart C-Pulse device is a surgically implanted system to help patients with moderate to severe HF. It utilizes the principles of intra-aortic balloon counter-pulsation applied in an extra-aortic approach to assist the LV. It reduces the workload required to pump blood throughout the body, while increasing blood flow to the coronary arteries. The vendor said combined, these potential benefits may help sustain the patient's current condition or, in some cases, reverse the heart failure process. This could potentially prevent the need for later-stage HF devices, such as LV assist devices (LVADs), artificial hearts or transplants. It may also provide relief from the symptoms of NYHA Class III and ambulatory Class IV HF and improve quality of life and cardiac function. Based on the results from the feasibility study, some patients treated with the C-Pulse System will be able to stop using the device due to sustained improvement in their conditions as a result of the therapy.
The system has CE mark approval in Europe and the company is seeking FDA approval. Results of its 20-patient FDA investigational device exemption (IDE) feasibility study[1] were published in the Journal of American College of Cardiology Heart Failure (JACC HF) last October. The study showed that the use of the C-Pulse System in Class III and ambulatory Class IV HF patients is feasible while providing preliminary indications of safety and improvements in functional status and health-related quality of life.
At six months, C-Pulse produced statistically significant improvements in NYHA class (3.1 to 1.9, p=0.0005). The six-minute walk distance improved at six months with statistically significant improvement at one year (289.7 to 336.5). Importantly, over the 12 months following initial placement of the device, only three of the 20 implanted patients (15 percent) had five adjudicated HF-related hospitalizations, none of which occurred within 30 days of implant.
“There are numerous challenges with the current treatment options of Class III and ambulatory Class IV heart failure patients. Surgical treatments available are often last resort, high-risk and invasive. The C-Pulse System addresses these challenges, is less invasive, has a lower risk profile and can be placed in patients too sick to be on optimal medical therapy alone, but not so sick that they require mechanical circulatory support or a heart transplant,” said William Abraham, M.D., director of the division of cardiovascular medicine at The Ohio State University Medical Center in a statement about the results.
Myocardial Anchoring
In patients with large infarcts following a heart attack, the LV can be reshaped using surgical ventricular reconstruction (SVR) to remove the scarred, non-functioning tissue. This requires stopping the patient’s heart and cardiopulmonary bypass while sections of the myocardium are removed. The BioVentrix Revivent myocardial anchoring system was introduced in Europe in 2014 as a minimally invasive alternative.
The system uses small titanium anchors placed along the outer surface of the heart and along one of the interior walls. The anchors are pulled toward one another, effectively excluding the scarred, and non-functioning heart wall. The ejection fraction efficiency of the remaining heart muscle is immediately improved, by as much as 30-40 percent, according to study data.
Reshaping the Heart With Injectable Gel
Last October, LoneStar Heart Inc. received European CE mark approval for its Algisyl-LVR hydrogel. The gel is intended to reverse HF progression in people who have an enlarged LV by surgically injecting it directly into the heart muscle. The hydrogel acts immediately as an internal scaffold that does not undergo long-term degradation and increases cardiac output. Preclinical studies show the gel decreases cardiac wall tension, while it improves heart muscle contractility and oxygen uptake, leading to a decrease in ventricle stress and to a marked cardiomechanic improvement.
LV Reshaping to Eliminate Mitral Regurgitation
Functional mitral valve regurgitation (FMR) is due to ventricular enlargement caused by HF. The current treatment is repairing the mitral valve using an open-heart surgical procedure on a bypass pump, which can be difficult to perform and risky for sicker patients. Even after successful repair surgery, FMR can reoccur because the ventricle often continues to get larger.
A minimally invasive surgical device may offer a new therapy option. First-in-human implants were made in 2014 using the Mardil Medical VenTouch ventricular reshaping device. The first two patients showed only trace amounts of FMR after implantation. The system’s delivery tool deploys the therapy device through a small incision between the ribs and guides it over the beating heart. The device is a biomaterial sleeve fitted with an inflatable, adjustable fluid chamber, which applies prescriptive pressure to a targeted location in order to realign valve leaflets, stopping the leaking by improving the shape and reducing the stress of the enlarged heart. If any further changes in the left ventricle occur after implantation, the VenTouch can be adjusted using a remote port.
“The VenTouch system is a promising technology that addresses both the annular and muscular abnormalities associated with FMR,” said William Cohn, M.D., FACS, FACCP, FAHA, director of minimally invasive cardiac surgery at the Texas Heart Institute in Houston, who helped with the procedures in Malaysia. “I was extremely impressed with the marked reduction in regurgitation we were able to demonstrate after a quick, off-pump procedure through a small incision.”
Pacemakers for HF
Vagus nerve stimulation (VNS) with electrical impulses from a pacemaker-like device is used to treat epilepsy and is being studied for numerous other neurological conditions. It is believed VNS might also help in controlling HF. Boston Scientific and BioControl Medical both developed VNS systems composed of a pacemaker pulse generator that is implanted under the skin with two connecting cuff electrodes that wrap around the vagus nerves on either side of the neck.
Last fall, BioControl Medical reached 480 randomized patients of its planned 650 patients for its INOVATE-HF (INcrease Of VAgal TonE in Heart Failure) trial of its CardioFit system. The trial includes more than 65 participating centers in the United States and Europe. Initiated in April 2011, INOVATE-HF is an IDE clinical study to determine the safety and efficacy of CardioFit in reducing hospitalization and death among patients with HF compared with standard evidence-based management. Results of the study will be used to support an FDA PMA.
Boston Scientific’s device data from the NECTAR-HF (NEural Cardiac TherApy foR Heart Failure) trial data, presented at the 2014 European Society of Cardiology (ESC) congress, failed to meet its six-month primary efficacy endpoint. It did not reduce left ventricular end systolic diameter as assessed by a blinded echocardiography core laboratory. However, quality-of-life metrics demonstrated significant symptomatic improvement despite the lack of a significant effect on primary and secondary endpoint measures of cardiac remodeling and functional capacity in HF patients. The study evaluated 96 NYHA Class II-III patients with an ejection fraction of less than 35 percent. All patients continued receiving optimal medical treatment for heart failure, but were randomized 2:1 to treatment or sham (implanted device, but not receiving therapy).
Reference:




 November 14, 2025
November 14, 2025 









