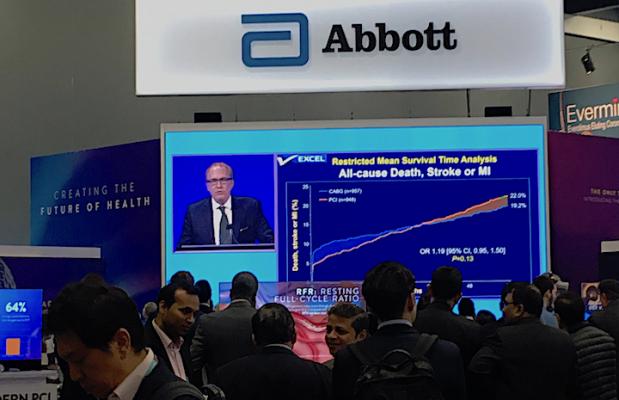
People watch the live presentation of the five-year EXCEL Trial data by Gregg Stone, M.D., in the Abbott booth at TCT 2019. Abbott makes the Xience stent used in the trial, which compared equally with long-term CABG surgical outcomes.
October 3, 2019 – Five-year data from the EXCEL Trial showed patients with left main coronary disease treated with percutaneous coronary intervention (PCI) using the Xience stent or coronary artery bypass graft surgery (CABG), had similar similar long-term, composite outcomes at five years.
Five-year findings were reported at the 2019 Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium. The study was also published simultaneously in the New England Journal of Medicine (NEJM).[1]
“In patients with left main coronary artery disease and low or intermediate coronary disease complexity, we found no significant difference between PCI and CABG with respect to the composite rate of death, stroke, or myocardial infarction at five years,” said lead investigator Gregg W. Stone, M.D., director of academic affairs for the Mount Sinai Health System, professor of medicine and professor of population health sciences and policy at The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, and co-director of medical research and education at the Cardiovascular Research Foundation (CRF). “Ten-year or longer follow-up is required to characterize the very late safety profile of PCI and CABG as both stents and bypass grafts progressively fail over time.”
The five-year data showed statistically the two therapies have about the same outcomes, but there was a data signal slightly favoring CABG.
Patients with left main coronary artery disease (LMCAD) typically have a poor prognosis due to the large amount of myocardium at risk. Revascularization with either PCI or CABG has been shown to prolong survival in patients with left main disease compared with medical therapy alone. Three-year data from the large-scale randomized EXCEL Trial found no significant difference in the composite rate of death, stroke or myocardial infarction (MI) between the two treatments, with a reduction in 30-day major adverse events with PCI. These results were first reported at TCT 2016 and published in NEJM.[2]
Between September 2010 and March 2014, 2,905 patients with LMCAD were recruited at 126 sites in 17 countries. Eligible patients (n=1,905) with LMCAD and site-assessed low or intermediate coronary artery disease complexity (SYNTAX score less than or equal to 32) were randomized to revascularization with fluoropolymer-based cobalt-chromium everolimus-eluting stents (EES; n=948) or CABG (n=957).
For the five-year analysis, the primary outcome was the composite of death, stroke or myocardial infarction. Long-term additional secondary outcomes included their components at five years, as well as therapy failure (definite stent thrombosis or symptomatic graft stenosis or occlusion), all revascularizations, and all cerebrovascular events (stroke or transient ischemic attack).
Five-year follow-up was achieved in 93.2% of patients receiving PCI and 90.1% of those who received CABG. The five-year primary composite of death, stroke, or myocardial infarction occurred in 22% of patients in the PCI group and 19.2% of patients in the CABG group (difference 2.8%; 95% CI -0.9% to 6.5%; P=0.13). The relative risk of PCI vs. CABG for the primary outcome varied between 0 to 30 days (HR 0.61, 95% CI 0.42 to 0.88), 30 days to one year (HR 1.07, 95% CI 0.68 to 1.70) and one year to five years (HR 1.61, 95% CI 1.23 to 2.12). Additional analyses demonstrated that the early benefit of PCI gradually diminished over time with increased post-procedural risk among patients randomized to PCI.
The five-year secondary composite of death, stroke, myocardial infarction, or ischemia-driven revascularization occurred in 31.3% of patients in the PCI group and 24.9% of patients in the CABG group (difference 6.5%; 95% CI 2.4% to 10.6%).
All-cause death occurred in 13% of patients in the PCI group and 9.9% of patients in the CABG group (difference 3.1%; 95% CI 0.2% to 6.1%). Eighteen of the 30 excess deaths in the PCI arm were adjudicated as non-cardiovascular deaths, five as definite cardiovascular deaths, and seven as undetermined cause. In addition, the five-year rates of stroke and myocardial infarction were not significantly different after PCI and CABG. Ischemia-driven revascularization within five years was performed more frequently after PCI than CABG, while the five-year rates of all cerebrovascular events and definite stent thrombosis or symptomatic graft stenosis or occlusion were less frequent with PCI than CABG.
The EXCEL study was funded by a research grant from Abbott Vascular. Stone has relationships with numerous device and pharmaceutical companies, but had no relevant disclosures for this study.
Related PCI vs. CABG Content:
VIDEO: Stents, Bypass Surgery Have Equal Outcomes in EXCEL Trial — Interview with David Kandzari, M.D.
Stents Have Equal Outcomes to Bypass Surgery in EXCEL Trial
Find information on other late-breaking TCT trials
References:


 January 05, 2026
January 05, 2026 









