The heart and kidneys are inextricably linked through a diverse web of hemodynamic, neural and hormonal mechanisms. As one organ fails, it pushes the other further away from physiological norms. The implication is that the heart is not the sole offender in
heart failure. In fact, in a study of more than 100,000 heart failure admissions, less than 10 percent of patients had normal renal function.[1]
When the kidneys are functioning properly, they maintain the fluid balance that keeps heart failure congestion in check. When venous congestion, lower arterial flow, and neurohormonal factors choke the filtration role of the kidneys, however, patients are unable to void an adequate volume of urine from their bodies, leading to worsening congestion and perpetuating the vicious cycle known as cardiorenal syndrome.
Congestion is responsible for almost all heart failure admissions but is often difficult to resolve due to poor kidney function. In fact, 90 percent of patients admitted with worsening renal function are discharged with persistent congestion and approximately one-third of these patients are back in the hospital or dead within two months.[2]
Cardiorenal syndrome is associated with twice the mortality of heart failure without renal dysfunction,[1] as well as increased length of stay and rehospitalization rates.[3] Despite these risks, there are no proven guidelines or therapies for cardiorenal patients. A large, unmet clinical need clearly exists to address cardiorenal syndrome.
The Economic Impact of Cardiorenal Syndrome
Cardiovascular disease accounts for the single biggest category of healthcare expenditure in the U.S. and the prevalence of heart failure is also expected to increase from 6.5 million to over 8 million Americans.[4] Already, the cost associated with treating heart failure is astronomical. In 2012, total annual cost for heart failure in the U.S. was estimated at $30.7 billion, and it is projected to rise to over $69.7 billion by 2030, or $244 for every adult.[4]
In the REMATCH study, we learned that medical therapy for heart failure costs $93,000 per year. For end-stage heart failure, the numbers are even more shocking. In addition to these ongoing medical costs, 10-month inpatient costs are $161,000 for a heart transplant or $230,000 for a mechanical left ventricular assist device (LVAD).[5]
Patients with end-stage heart failure consume a disproportionate amount of heart failure resources in their last six months of life. As mentioned above, select patients with end-stage heart failure may receive an LVAD to replace the function of the left heart. Whether or not a technology is cost-effective is often described using cost per quality-adjusted life year ($/QALY), a measure that takes into account both quality and quantity of life added. A generally accepted threshold value is $50,000/QALY, meaning payers spend $50,000 to add one high-quality year to a patient’s life. However, economic studies show that LVADs cost over $200,000/QALY.[6]
More than 5,000 LVADs are placed in the U.S. each year, more than the total number of heart transplants. According to research from the Department of Surgery, University of Virginia Health System, the approximate nationwide annual expenditures for LVAD procedure admissions more than tripled, from $143 million in 2005 to $479 million in 2009. Cost and socio-economic status are major factors driving the widening chasm between the 600,000 Americans with advanced heart failure and poor quality of life, versus the small number who receive LVADs and/or heart transplants. Oxford University Press suggests future strategies to reduce costs of heart failure should primarily focus on the reduction of hospitalization, which represents the largest part of treatment costs.
A Tiny Solution to Huge Heart Failure Costs
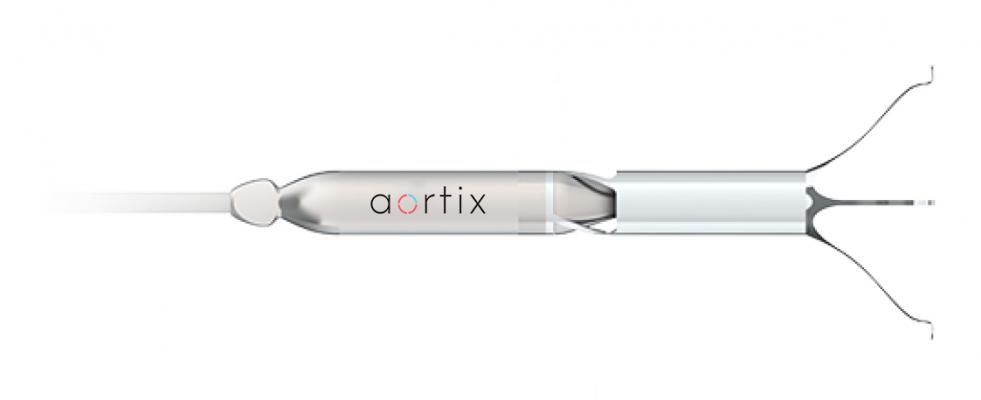
Houston-based medical device company Procyrion is working on a device designed to treat heart failure and reduce hospitalizations with a quick, cath-lab implant procedure. Aortix is an intra-aortic axial flow pump designed to unload the heart and increase renal perfusion. Due to its unique deployment location in the descending thoracic aorta and close proximity to the kidneys, a distinct opportunity exists to address the problems associated with cardiorenal syndrome by improving perfusion to the kidneys in addition to unloading the heart.
Unlike LVADs and other costly devices requiring highly invasive, high-risk surgical implantation, the Aortix device is deployed via a femoral catheter to the descending aorta where nitinol anchors expand and affix the pump to the wall of the aorta. At 6 mm in diameter (thinner than a #2 pencil), the device can be implanted in a percutaneous cath-lab procedure in under 10 minutes. The reduction in procedure time and potential for a reduction in length of stay alone could greatly reduce the cost of care in these patients.
Aortix is initially intended to be left in the aorta for three to seven days in order to quickly and completely decongest patients while resting the heart, which should translate to a more rapid discharge from the hospital. Additionally, studies have shown that patients who are discharged from the hospital decongested have reduced mortality and readmissions up to one-year out.[7,8]
Once implanted, a thin, flexible electrical lead is connected to an external motor controller. Once activated, the pump operates at a speed around 25,000 RPM, depending on the level of support required. The jet pump entrains native aortic blood flow, transferring energy to the surrounding blood and creating a pressure gradient across the pump. Downstream of the pump, the kidneys see increased pressure and flow, which leads to improved kidney function with increased urine output and decreased fluid overload. Upstream of the pump, Aortix reduces the pressure the heart has to pump against, unloading the heart. This pressure relief reduces the overall workload of the heart and improves cardiac output, further disrupting the cardiorenal syndrome and improving downstream perfusion.
If a low risk, minimally invasive device such as Aortix is effective at decongesting the patient in-hospital and reducing heart failure rehospitalization, it could dramatically shift the standard of heart failure care. Instead of continuing to spend disproportionate resources at the end of the continuum of care, a cardiorenal support device could delay patients from reaching end-stage by giving the kidneys the perfusion they need to combat congestion and slow the progression of heart failure.
Early Clinical Work Confirms Potential for Cardiorenal Therapy
The first-in-human experience with the Aortix pump (n=6), recently published in
Catheter and Coronary Interventions, details a short-term use of the Aortix pump (47-95 min) in high-risk patients with decreased renal function undergoing percutaneous coronary interventions (PCIs).[9] Aortix was delivered through an 18 French sheath in a percutaneous procedure. Implantation took between 4-9 minutes and the mean flow rate through the pump was 3.5 L/min.
During pump support, the mean rate of urine output increased 10x (range 2.5-25.0x). Estimated glomerular filtration rate (eGFR) also increased at discharge when compared to baseline (6.95 ± 8.09 mL/min). No device failures occurred and the PCI procedure was successful in all patients. There were no adverse events, hemolysis, hemodynamic compromise, or vascular complications. This dramatic effect on the kidneys points toward a potential renal-protective effect, reversing the negative effects of cardiorenal syndrome.
Cardiorenal syndrome is a major cause of heart failure morbidity and mortality, playing a role in the rising cost of the heart failure epidemic.
Without adequate treatment options, patients experience an extremely poor quality of life, struggling to breathe, enduring painful swelling and they need to severely limit activity. In order to remedy this condition, a solution must address the source of the problems at both the heart and the kidneys. Procyrion’s Aortix pump aims to curtail the progression of heart failure by fully decongesting decompensated patients, reducing costs and hospitalizations, and minimizing the need for expensive, end-stage surgical solutions.
Editor’s note: Author Will Clifton, M.D., is a bioengineer with a background in medical device and innovation education, and currently serves as director of the Global Medical Innovation track in the master of bioengineering program at Rice University, Houston, Texas. He is a clinical consultant for Procyrion Inc., the company developing the Aortix device. The Aortix is in the research and development phase and is not approved for sale in any country. For more information, visit www.procyrion.com.
Related Content on the Aortix Pump:
References:



 November 14, 2025
November 14, 2025 









