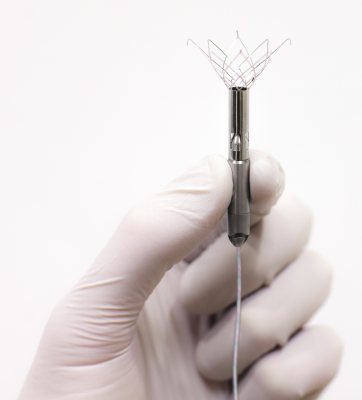
February 7, 2014 — Start-up company Procyrion Inc. is developing a catheter-deployed circulatory assist
device intended for long-term use in the treatment of chronic heart failure. The 6-mm diameter Aortix device is narrower than a pencil and is delivered via a catheter in a minimally invasive outpatient procedure lasting about 10 minutes.
Currently, long-term mechanical circulatory support (MCS) is limited to large
ventricular assist devices (VADs). These devices are mainly used in patients with late stage
heart failure (HF). Procyrion is developing a percutaneous intra-aortic micro-axial fluid entrainment pump called Aortix intended for long-term MCS in patients with earlier stage HF.
Aortix has been successfully tested with computer models, on the bench top in mock flow loops and in vivo in large animal HF studies. In three porcine experiments conducted last year, the pump was deployed in the thoracic aorta by standard cardiac catheterization techniques and was anchored with self-expanding struts. Acute cardiac dysfunction was induced by infusing esmolol continuously. Pump support increased cardiac output (+10.4 percent), stroke volume (+8.9 percent), and ejection fraction (+10.8 percent) while decreasing cardiac stroke work (-10.8 percent) and afterload (-22.7 percent). Pump support enhanced renal perfusion through sustained increases in both renal artery flow (+36.4 percent) and pressure (+73.6 percent).
In a porcine model of acute HF, the catheter-based intra-aortic fluid entrainment pump improved hemodynamics and renal perfusion. These results suggest the pump could improve HF outcomes and patients' quality of life by resting the heart, promoting reverse remodeling and augmenting end-organ perfusion. The renal perfusion may help disrupt the cardiorenal syndrome cycle and improve HF treatment.
The company received the Commercialization award by the Gulf Coast Regional Center of Innovation and Commercialization in February 2013 and received a total of $1.5 million in assistance from the Texas Emerging Technology Fund.
For more information: www.procyrion.com