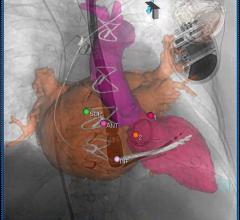
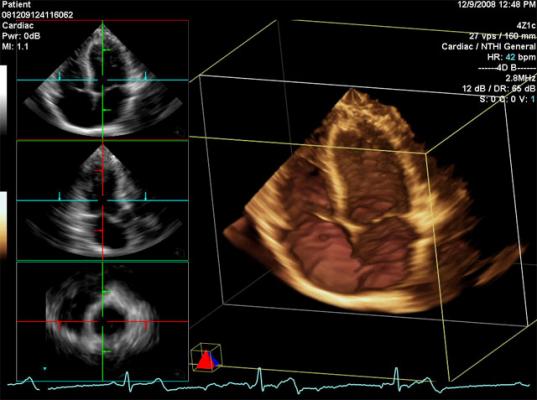
July 6, 2012 — With last year’s U.S. Food and Drug Administration approval of transcatheter aortic valve replacement (TAVR) and increasingly complex cath lab and hybrid operating room (OR) interventions, ultrasound is playing an increasing role in procedural guidance. This is especially true for use of 3-D/4-D transesophageal echo (TEE), which enables the more precise surgical-view visualization required for structural heart procedures, such as atrial septal defect (ASD) occluder and MitraClip deployments. These repairs would otherwise be extremely difficult to accomplish using fluoroscopy alone.
As complex invasive surgical procedures increasingly convert to minimally invasive interventional procedures, the rapid rise in TEE is creating a new niche specialty of interventional echocardiography, said several experts who presented this week at the American Society of Echocardiography (ASE) 2012 meeting. Among the packed sessions were those involving TEE use for interventional procedures, especially transcatheter valve replacements.
“With the increased interest in structural heart disease we are depending much more on techniques like TEE, and actually this whole area of interventional echocardiography is really on the rise,” said Malissa Wood, M.D., co-director of the Women’s Center at Massachusetts General Hospital, Boston. She also serves as the chair of the ASE’s Public Relations Committee. She noted an increased focus on this area of echo at this year’s ASE.
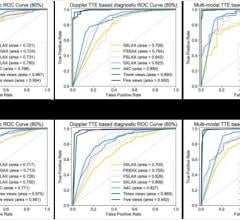
The value of TAVR depends on careful selection of patients who, while not surgical candidates, do not have such extreme comorbidities that overwhelm the benefits of TAVR and render the intervention futile. In selection of these patients, Doppler echocardiography has now replaced cardiac catheterization as the gold standard diagnostic technique for quantifying aortic stenosis (AS). A transthoracic echocardiogram (TTE) using an external probe on the patient’s chest can determine whether the AS is severe enough for TAVR. TEE supplements the information from TTE, providing precise measurements of the aortic valve annulus, critical for selecting the proper size of the implanted valve. Color Doppler TEE is used to determine the amount of regurgitation both pre- and post-procedurally and to check for paravalvular leaks. TEE is also widely used to guide device placement during the TAVR procedure.
New TEE Technology
GE Healthcare showcased its recently released Vivid E9 Breakthrough 2012 (BT12) cardiac ultrasound system, which includes a 3-D/4-D TEE probe. It offers tools to improve workflow efficiency through simplified image acquisition. It is designed for intuitive navigation and advanced, yet easy-to-use, quantification.
“With a recent increase in TEE procedures, our customers in the cath lab, echo lab and operating room have been looking for ways to achieve the benefits of 4-D imaging without compromising productivity,” said Al Lojewski, general manager of cardiovascular ultrasound at GE Healthcare. “Our Vivid E9 system was designed to help make 4-D imaging easier and more efficient.”
The new transesophageal probe allows 4-D dataset acquisitions to quickly visualize cardiac structures. It also can help quantify left ventricular pump function, myocardial deformation and mitral valve morphology.
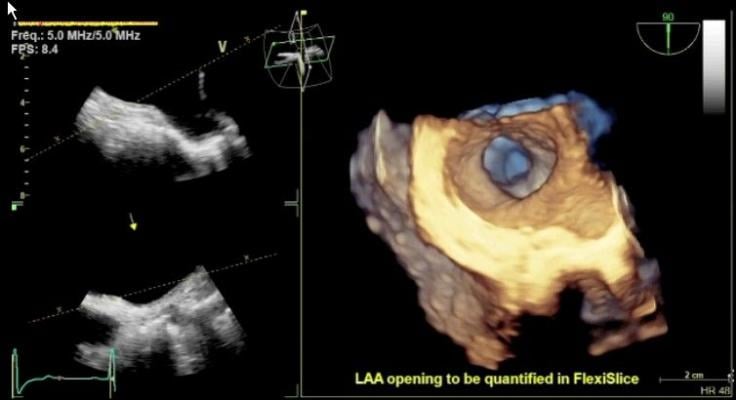
The 4-D TEE probe includes triplane imaging, 4-D views and high frame rates. The system enables one-button acquisition of mitral valve images. In biplane mode, it has the ability to tilt and rotate at the same time, which aids in the accurate placement of devices such as the MitraClip. A new QuickRotate button provides consistency and accuracy by allowing users to rotate an image in 30-degree increments with a single click. The system also offers laser lines, a new tool to help understand the relationship between 2-D slices and 4-D views, helping visualize the linkage between 2-D and 4-D and improve depth perception.
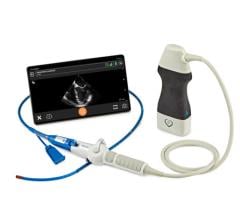
Philips announced that the new CX50 xMatrix portable ultrasound will now interface with its Live 3D TEE. Previously only available with the larger iE33 xMatrix system, the system offers a smaller footprint for easier use and movement in a cath lab or hybrid OR.
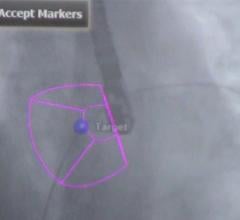
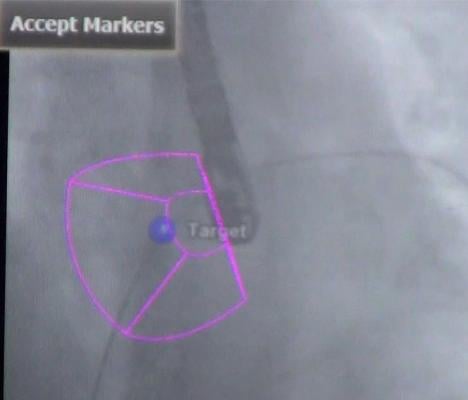
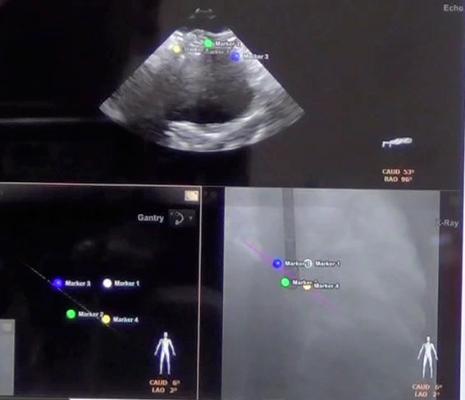
The CX50 xMatrix architecture is designed to support future capabilities, including advanced fluoro integration. At ASE, Philips also unveiled EchoNavigator, a 510(k)-pending system that aligns TEE views with live fluoroscopy. The user can mark locations on a live TEE image, and then marks precisely light up to show the location and image orientation on live fluoroscopy for better procedural navigation.
Intra-Cardiac Echo (ICE)
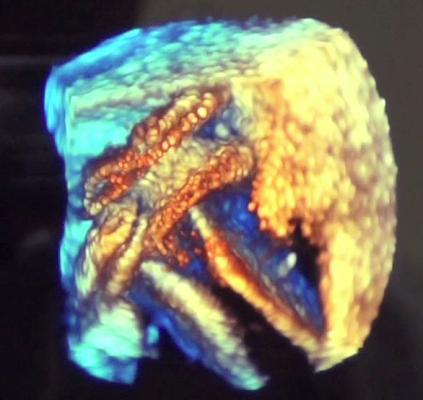
Released earlier this year, at ASE Siemens highlighted version 2.0 of its Acuson SC2000 cardiac ultrasound system. While offering improved workflow and several new applications, an innovation that could have a big impact on interventional structural heart and electrophysiology (EP) procedures is the system’s introduction of volumetric, 3-D intra-cardiac echocardiography (ICE). It integrates with the new Acuson AcuNav V ultrasound catheters currently pending regulatory review.
Its 3-D cine volumes can be rotated to better visualize the anatomy inside the heart and help guide procedures. The image offers an accurate model of the interior of the heart, which may offer an alternative to visualizing ablation procedures, instead of relying on a computer model created by an EP electro-mapping system. Siemens said it is developing an EP ablation scar mapping system that will overlay on its 3-D ICE images.
Technology is Saving Time
One of the biggest complaints from end-users regarding advanced cardiac ultrasound technology is the large amount of time required to manipulate 3-D images or use quantification tools offered by advanced visualization software. In response, vendors have released increasingly advanced software in recent years that automates many functions and reduces the number of clicks and processing time required. Several of these improvements were highlighted by vendors on the ASE show floor.
“The newer 3-D/4-D platforms are much more user-friendly, and hospitals, not just academic medical centers, can now use and access these techniques and do a 3-D/4-D exam in a short amount of time,” Wood said.
She said newer software on the echo machines has helped reduce the time required for a 3-D/4-D scan by about half. For speckle tracking, torsion, strain and tissue Doppler, Wood said the time has been cut by about a third.
At ASE, Philips introduced its new CX50 xMatrix Vision 2013 software upgrade. It includes Qlab 9.0 with an improved speckle tracking algorithm for faster, more robust strain quantification and improved annotations and workflow.
GE's Vivid E9 BT12 includes several features to enhance 4-D imaging and workflow. These include two-click crop.
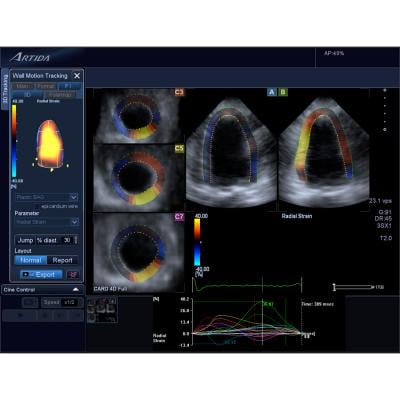
Toshiba showcased new 3.0 software on its Aplio Artida. It offers improved 3-D imaging and next-generation 3-D Wall Motion Tracking for accurate and reproducible quantitative analysis of cardiac function.
Siemens showed its Acuson SC2000 ultrasound system with single-beat, full-volume imaging that easily integrates with standard 2-D protocols. It also explained how its technologies – including eSie LVA volume LV analysis, eSie PISA volume analysis, eSie Echo workflow applications – generate results regardless of the difficulty of the case.
To see a video interview with Wood discussing trends at ASE 2012, click here.


 June 13, 2024
June 13, 2024