May 9, 2017 — Biotronik announced U.S. Food and Drug Administration (FDA) approval and the launch of Sentus ProMRI, the ...
Cardiac Resynchronization Therapy Devices (CRT)
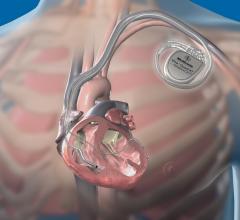
Cardiac Resynchronization Therapy Devices (CRT) are cardiac electrophysiology (EP) systems that include a small electronic device that is surgically implanted into a pocket of skin to help both ventricles contract together. They are also called biventricular pacemakers. These systems can be used for pacing only, or can include a built-in defibrillator.
May 9, 2017 — The U.S. Food and Drug Administration (FDA) has cleared Boston Scientific’s Resonate family of implantable ...
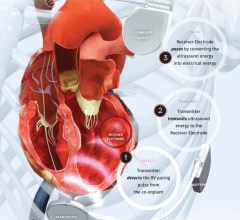
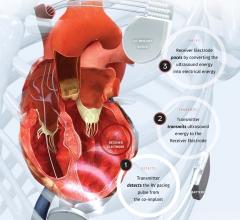
May 12, 2017 – New study proves safety of novel, wireless pacing system, WiSE CRT (Wireless Stimulation Endocardially ...
April 14, 2017 — The National Institute for Health and Care Excellence (NICE) recently issued medical technology ...
March 23, 2017 — Biotronik announced the European launch of the Edora series, its smallest series of pacemakers and card ...
March 7, 2017 — Medtronic plc recently announced an economic analysis of five-year data showing that patients with mild ...

January 9, 2017 — The U.S. Food and Drug Administration (FDA) issued a safety communication today concerning patient ...
November 15, 2016 — Medtronic plc has received U.S. Food and Drug Administration (FDA) approval for the Claria MRI Quad ...
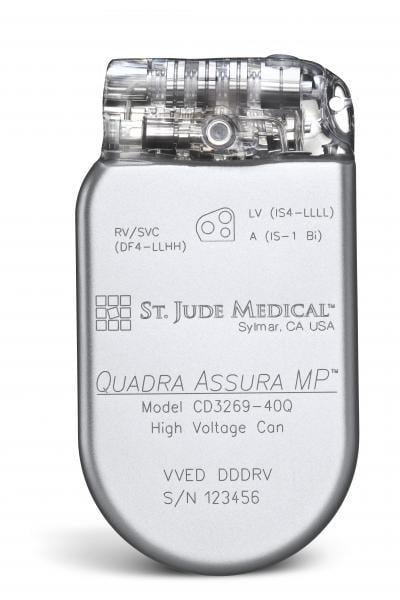
October 25, 2016 — St. Jude Medical is recalling 251,346 of its Fortify, Unify and Assura implantable cardioverter ...
October 13, 2016 — Medtronic plc is the first company to receive U.S. Food and Drug Administration (FDA) approval for ...
September 27, 2016 — In August, the European Heart Rhythm Association (EHRA) and EP Europace journal announced the ...
September 20, 2016 — Medtronic plc announced the results of an analysis that reveals patients increasingly adhere to hea ...
September 15, 2016 — EBR Systems Inc. announced the U.S. Food and Drug Administration (FDA) has granted an ...
September 7, 2016 — St. Jude Medical Inc. (SJM) has filed a lawsuit against Muddy Waters Consulting LLC, Muddy Waters ...
August 30, 2016 — Medtronic plc announced results from the Cardiac Resynchronization Therapy Efficacy Enhancement (CRTee ...

 May 09, 2017
May 09, 2017