July 21, 2025. — Electrophysiologists at the Texas Cardiac Arrhythmia Institute (TCAI) at St. David's Medical Center recently became the first in the nation to implant an FDA-approved novel leadless ...
Cardiac Resynchronization Therapy Devices (CRT)
Cardiac Resynchronization Therapy Devices (CRT) are cardiac electrophysiology (EP) systems that include a small electronic device that is surgically implanted into a pocket of skin to help both ventricles contract together. They are also called biventricular pacemakers. These systems can be used for pacing only, or can include a built-in defibrillator.
July 21, 2025. — Electrophysiologists at the Texas Cardiac Arrhythmia Institute (TCAI) at St. David's Medical Center ...
April 25, 2025 – Findings from two late-breaking clinical trials demonstrate the safety and efficacy of left bundle ...
June 25, 2024 — Heart Hospital of New Mexico at Lovelace Medical Center (HHNM) and GE HealthCare (announced HHNM as the ...
January 11, 2024 — It is with great sadness that the Diagnostic and Interventional Cardiology (DAIC) team has learned of ...
July 18, 2023 — The U.S. Food and Drug Administration (FDA) has issued a Class I recall on the Medtronic ICDs and CRT-Ds ...
May 22, 2023 — Results from a pivotal clinical trial found a leadless pacemaker can deliver cardiac resynchronization ...
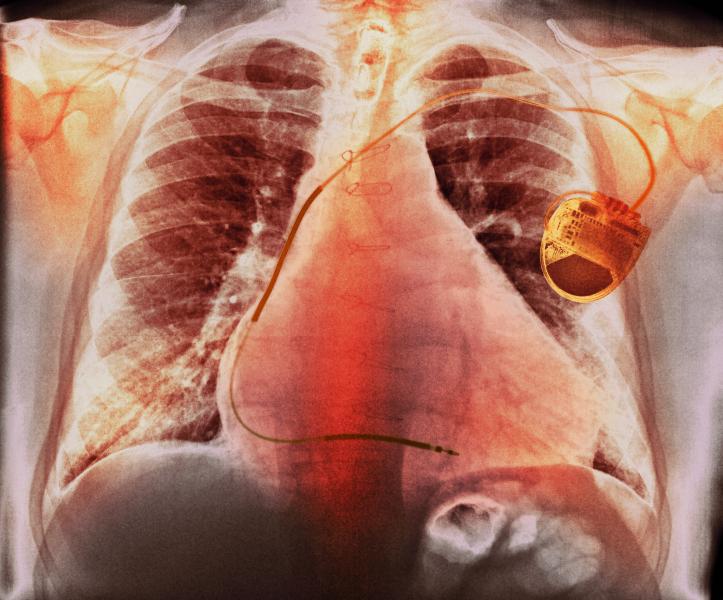
Arrhythmias can be described as irregular heart rhythms that are brought on by numerous factors, including heart damage ...
August 19, 2022 — According to a release just issued by the U.S. Food and Drug Administration (FDA), Medtronic is ...
Royal Philips, a global leader in health technology, announced late-breaking results of a large-scale, real-world ...

November 1, 2021 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
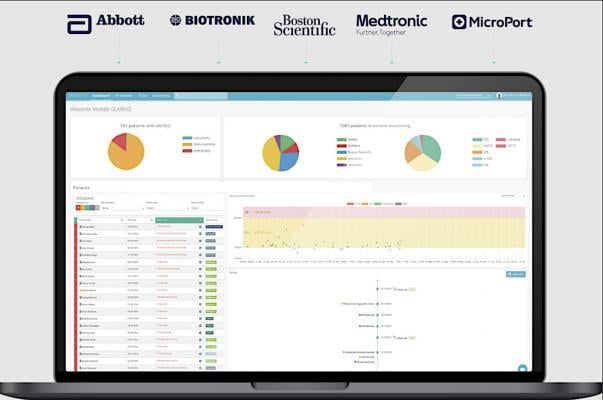
With 1.2 to 1.4 million new electrophysiology (EP) devices being prescribed to patients around the world each year ...
October 27, 2021 — EBR Systems Inc., developer of the world’s first wireless cardiac pacing system for heart failure, ...
October 1, 2021 — The European Society of Cardiology (ESC) guidelines on cardiac pacing and cardiac resynchronization ...
Khaldoun Tarakji, M.D., MPH, associate section head, cardiac electrophysiology, Heart and Vascular Institute at ...




 July 21, 2025
July 21, 2025