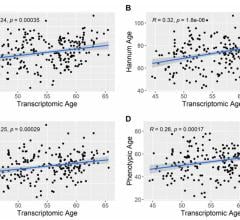
May 22, 2024 — New research from The Cooper Institute and partners at University of Texas (UT) Southwestern Medical ...
Cardiovascular Clinical Studies
This channel includes news and new technology innovations from cardiovascular clinical trials. These clinical studies include all cardiac subspecialties.
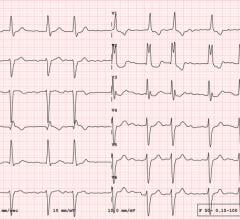
May 23, 2024 — A newly-published peer-reviewed study in the Journal of the American Heart Association, JAHA, found that ...
May 21, 2024 — A new research paper was published in Aging (listed by MEDLINE/PubMed as "Aging (Albany NY)" and "Aging ...
May 20, 2024 — Cardiologist Brendan Carry, MD, and a team of Danville, PA-based Geisinger Medical Center physicians have ...
May 19, 2024 — A physician-initiated prospective, randomized clinical trial sponsored by the National Heart, Lung, and ...
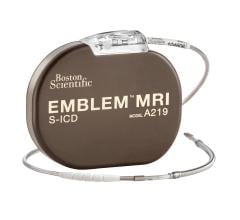
May 18, 2024 — Boston Scientific Corporation today announced positive six-month results from the ongoing pivotal MODULAR ...
May 18, 2024 — The Heart Rhythm Society (HRS) has announced the findings of three new studies demonstrating the safety ...
May 17, 2024 — Royal Philips, a global leader in health technology, is presenting new retrospective study results ...
May 17, 2024 — The study design and baseline characteristics of FINE-HEART, a new prespecified, exploratory pooled ...
May 16, 2024 — People were more likely to develop a type of treatment-resistant hypertension when they experienced ...
May 16, 2024 — A recent publication in the American Heart Association Circulation highlights a comprehensive ...
May 16, 2024 — A new University of Cincinnati study provides more insight into how few patients have severe ischemic ...
May 15, 2024 — A new study demonstrated parity between a minimally invasive procedure to replace the aortic valve in the ...
May 14, 2024 — One of the most common genetic heart diseases worldwide, hypertrophic cardiomyopathy (HCM) causes the ...


 May 22, 2024
May 22, 2024