Chandan Devireddy, M.D., offers insights about what he saw as the top take aways from the 2019 Transcatheter ...
Drug-Eluting Balloons
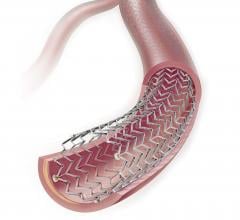
This channel includes news and new technology innovations for drug-coated balloons (DCB), also referred to as drug-eluting balloons. These are used to treat peripheral and coronary artery lesions and restenosis. The balloons carry an antiproliferative drug that is delivered to the wall of arteries when the balloon is expanded. The drug helps prevent neointimal hyperplasia (scar tissue growth) caused by trauma when the vessel segment is treated for atherosclerotic lesions with balloon angioplasty. DCBs can be used to treat hyperplasia in arteriovenous (AV) access fistulae in dialysis patients, where the vessel undergoes repeated trauma from regular punctures. DCBs also are used to treat in-stent restenosis due to scar tissue proliferation inside stents, which can cause a vessel to occlude.
September 20, 2019 — Orchestra BioMed Inc., in partnership with Terumo Corp. announced the company has secured ...
August 27, 2019 — Concept Medical was recently granted Breakthrough Therapy designation by the U.S. Food and Drug ...
August 14, 2019 — Concept Medical Inc. (CMI) has been granted "Breakthrough Device Designation" from the U.S. Food and ...
August 2, 2019 — B. Braun Interventional Systems Inc. (BIS) announced the U.S. Food and Drug Administration (FDA) has ...
June 13, 2019 — Orchestra BioMed Inc. announced it has formed a global strategic partnership with Terumo Corp. for ...
May 29, 2019 — Philips announced the three-year results from the ILLUMENATE Pivotal trial and the ILLUMENATE European ...
May 2, 2019 — Concept Medical Inc. (CMI) has been granted Breakthrough Device Designation from the U.S. Food and Drug ...
April 24, 2019 — Orchestra BioMed Inc. has secured Breakthrough Device designation by the U.S. Food and Drug ...
March 6, 2019 – Swiss-based M.A. MedAlliance SA has been granted Breakthrough Device Designation from the U.S. Food and ...
January 28, 2019 — Philips announced the latest pooled analysis of patient-level data of over 2,300 patients treated ...
January 17, 2019 — The U.S. Food and Drug Administration (FDA) issued a letter Jan. 17, 2019, to healthcare providers ...
September 25, 2018 — Orchestra BioMed Inc. announced the three-year clinical results from its Sirolimus Angioplasty ...
September 24, 2018 — New data announced at the 2018 Transcatheter Cardiovascular Therapeutics (TCT) conference, Sept. 21 ...
June 15, 2018 — Medtronic plc has received U.S. Food and Drug Administration (FDA) approval for 200mm and 250mm lengths ...


 October 04, 2019
October 04, 2019