Juan F. Granada, M.D., CEO of the Cardiovascular Research Foundation (CRF) worked on preclinical development work for a ...
Drug-Eluting Balloons
This channel includes news and new technology innovations for drug-coated balloons (DCB), also referred to as drug-eluting balloons. These are used to treat peripheral and coronary artery lesions and restenosis. The balloons carry an antiproliferative drug that is delivered to the wall of arteries when the balloon is expanded. The drug helps prevent neointimal hyperplasia (scar tissue growth) caused by trauma when the vessel segment is treated for atherosclerotic lesions with balloon angioplasty. DCBs can be used to treat hyperplasia in arteriovenous (AV) access fistulae in dialysis patients, where the vessel undergoes repeated trauma from regular punctures. DCBs also are used to treat in-stent restenosis due to scar tissue proliferation inside stents, which can cause a vessel to occlude.

Drug-coated balloons (DCB), also referred to as drug-eluting balloons (DEB), were created as a way to reduce very high ...
July 7, 2020 – MedAlliance announced enrollment of the first patient in its study of Selution SLR 0.014 drug-eluting ...
Drug-coated balloons (DCB), also referred to as drug-eluting balloons, are used to treat peripheral and coronary artery ...
June 19, 2020 — iVascular SLU announced the global launch of Essential Pro, a novel coronary artery drug-coated balloon ...
June 17, 2020 — MedAlliance announced its second CE mark approval for its Selution SLR 0.014 percutaneous transluminal ...
May 28, 2020 – MedAlliance has announced the award of its second European CE mark clearance for its Selution SLR 0.014 ...
November 21, 2019 — The U.S. Food and Drug Administration (FDA) has cleared the Medtronic In.Pact AV drug-coated balloon ...
November 7, 2019 — The IN.PACT Arteriovenous (AV) Access randomized trial evaluating the safety and effectiveness of the ...
November 7, 2019 — Real-world, four-year study results of the In.Pact Admiral drug-coated balloon (DCB) demonstrate long ...
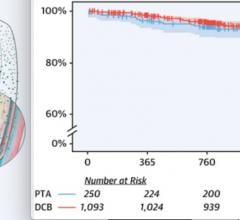
November 7, 2019 — There was no difference between drug-coated balloons (DCB) vs. plain old balloon angioplasty (POBA) ...
November 6, 2019 – Positive, two-year data from the first-in-human study of Selution SLR, MedAlliance’s novel sirolimus ...
November 6, 2019 – Boston Scientific announced positive data for two of its devices within the peripheral drug-eluting ...
October 15, 2019 — Royal Philips introduced two new balloons to its Stellarex 0.035-inch low-dose drug-coated balloon ...
October 10, 2019 — An independent analysis of Lutonix 035 drug-coated balloon (DCB) patient-level data showed no ...


 September 09, 2020
September 09, 2020