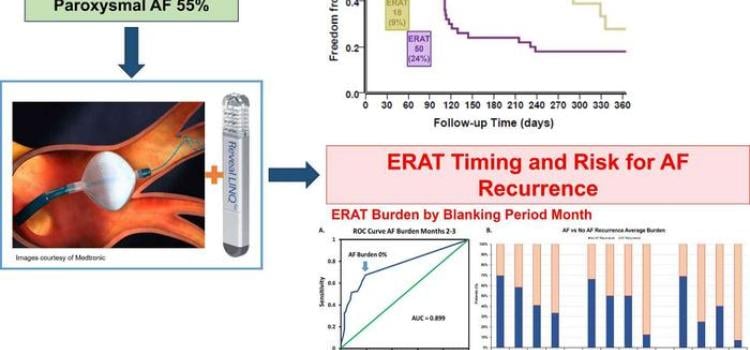
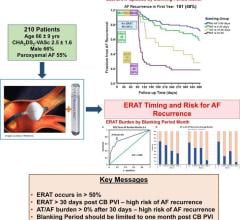
April 18, 2024 — New evidence-based research calls into question the conventional three-month blanking period immediately after atrial fibrillation (AF) ablation, when early occurrences of AF are ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.
April 18, 2024 — New evidence-based research calls into question the conventional three-month blanking period ...
April 10, 2024 — An international consensus statement on how to treat atrial fibrillation with catheter or surgical ...
April 5, 2024 — Johnson & Johnson and Shockwave Medical, Inc. today announced that they have entered into a definitive ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...

Here is a Top 10 look at the content that was trending during the month of March on dicardiology.com:
1. CLS Health ...
March 28, 2024 — Biosense Webster, Inc., a global leader in cardiac arrhythmia treatment and part of Johnson & Johnson ...
March 26, 2024 — UC San Diego Health is the first health system in San Diego to successfully implant the world’s first ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...

The American College of Cardiology (ACC) has announced key educational and programming highlights for its ACC 73rd ...
March 18, 2024 — Conformal Medical, Inc. announced that the CLAAS System was featured in a podium presentation at the Ca ...
March 15, 2024 — Following the recent CE approval of the Solia S lead1 for LBBAP, BIOTRONIK proudly announces the world ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
March 15, 2024 — Following the recent CE approval of the Solia S lead1 for LBBAP, Biotronik announces the world’s first ...

February was a short but busy month in the diagnostic and interventional cardiology world, with a lot of news being ...
February 27, 2024 — Biosense Webster, Inc., a global leader in cardiac arrhythmia treatment and part of Johnson & ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
PLEASE NOTE: This webinar has been postponed to a later date.
A new date will be posted in the coming days.
...
February 16, 2024 — AMO Pharma Limited, a privately held clinical-stage specialty biopharmaceutical company focusing on ...
February 15, 2024 — Canary Medical, a medical data company focused on the development and commercialization of its ...




 April 18, 2024
April 18, 2024