Children’s Hospital Los Angeles cardiologist Michael Silka, M.D., helped to pioneer the development of indications for ...
EP Lab
This channel includes news and new technology innovations for cardiac electrophysiology (EP) systems, techniques and devices using in EP labs. This includes implantable EP devices, pacemakers, implantable cardioverter defibrillators (ICD), cardiac resychronization therapy (CRT), ablation technologies, left atrial appendage (LAA) occlusion, atrial fibrilation (AF) and Holter monitors.

Improved short-term monitoring methods for patients with stroke risk can increase early detection of atrial fibrillation ...

December 3, 2021 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) m ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
November 22, 2021 — BioSig Technologies Inc., a medical technology company commercializing an innovative biomedical ...
Christine Albert, M.D., MPH, professor, chair of the Department of Cardiology and the Lee and Harold Kapelovitz ...
November 18, 2021 — The U.S. Food and Drug Administration (FDA) is reminding providers about the risk of major ...
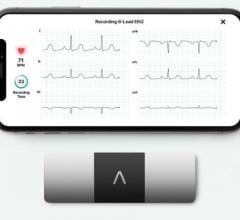
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
November 17, 2021 — People with atrial fibrillation (AFib) who underwent individualized testing to discover triggers for ...
November 16, 2021 — Acutus Medical Inc. (Acutus), an arrhythmia management company focused on improving the way cardiac ...
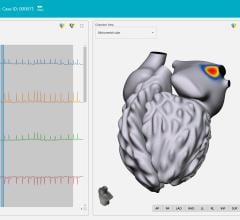
November 15, 2021 — Vektor Medical Inc. announced U.S. Food and Drug Administration (FDA) 510(k) clearance for its ...
Washington Health System (WHS) provides healthcare services at more than 40 offsite locations across three counties in ...
November 12, 2021 — Orchestra BioMed Inc. announced multiple presentations of long-term clinical results and ISH ...
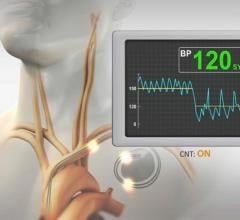
November 2, 2021 — Cardionomic Inc. announce initial U.S. enrollment in its global Cardiac Pulmonary Nerve Stimulation ...

November 1, 2021 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
Apple created a stir when it announced in 2018 that its Apple Watch Series 4 was the first consumer health and fitness ...
With 1.2 to 1.4 million new electrophysiology (EP) devices being prescribed to patients around the world each year ...
October 28, 2021 — CathVision, a medical technology company developing electrophysiology (EP) solutions in EP recording ...
October 27, 2021 — Impulse Dynamics announced the U.S. Food and Drug Administration approved a modification of labeling ...


 December 07, 2021
December 07, 2021

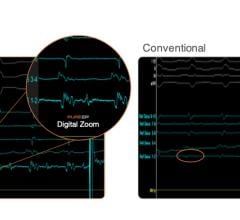



![Figures from an initial study on the Cardionomic CPNS technology in a poster presentation at the Heart Rhythm Society (HRS) 2021 meeting.[1] Starting at top left, an angiographic view of the atrial transseptal access for a left ventricular septal ablation. Procedural intra-cardiac echo (ICE) showing ablation catheter positioning. A 3D electro-anatomic map of the LV septum. The final graphs show baseline and post-procedure LVOT pressure readings demonstrating a decreased gradient.](/sites/default/files/styles/content_feed_medium/public/Cardionomic_Cardiac_Pulmonary_Nerve_Stimulation_CPNS_for_HF.jpg?itok=ecrU9hmQ)