September 12, 2014 — Volcano Corp. announced it will be launching its interventional precision guidance tools at the ...
FFR Technologies
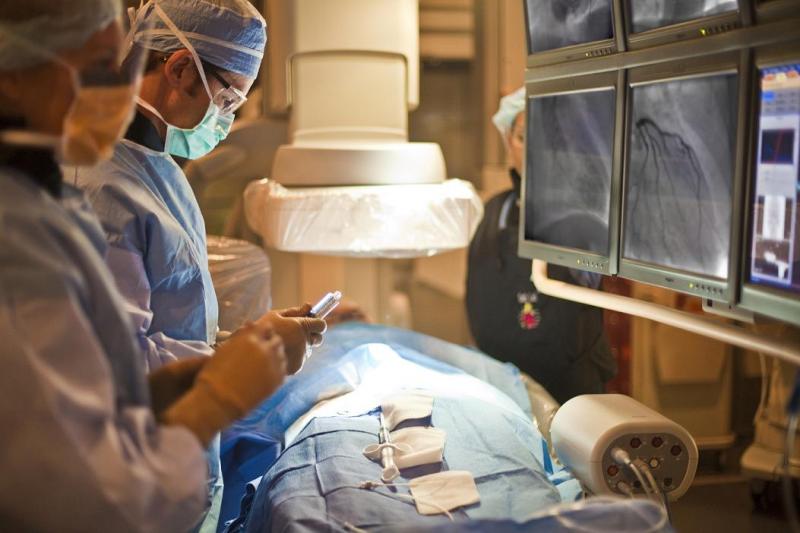
This channel includes news and new technology innovations for fractional flow reserve (FFR) wires, catheters and systems used to measure blood flow across a coronary lesion to determine if a stent is needed or if the plaque stenosis can be treated medically. The section includes iFR, instantaneous wave-free ratio, systems used in the cath lab and noninvasive FFR technologies including computed tomography-FFR. This is also referred to as CT-FFR or FFR-CT.
September 4, 2014 — St. Jude Medical announced a new multicenter clinical trial has found that using the company’s fract ...
August 20, 2014 — Boston Scientific Corp. and Asahi Intecc have formalized plans to develop a new, differentiated fracti ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...

August 6, 2014 — Acist Medical Systems announced it entered into a strategic agreement with Medtronic to co-promote the ...
July 24, 2014 — Boston Scientific has initiated full commercial launch of its new Polaris imaging system. This system ...
June 5, 2014 — New measures of the severity of coronary artery blockages do not provide enough accuracy to guide ...
April 22, 2014 — Volcano Corp. announced U.S. Food and Drug Administration (FDA) clearance of its proprietary Instant ...
April 2, 2014 — Acist Medical Systems Inc. announced at the American College of Cardiology's 63rd Annual Scientific ...

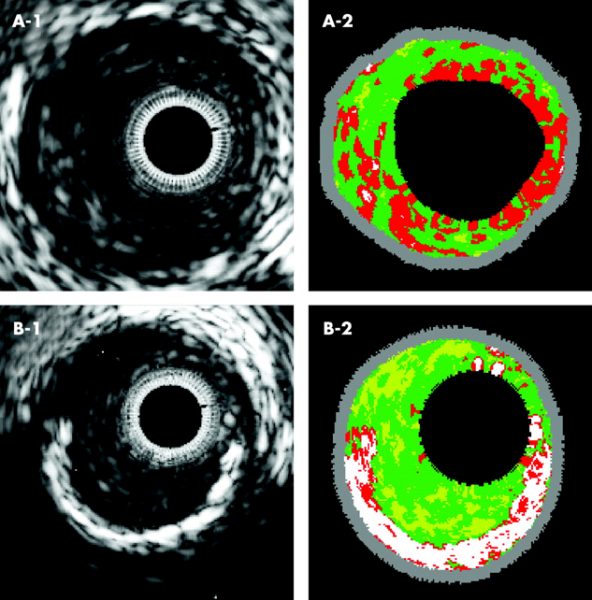
By Dave Fornell, DAIC editor
The Cardiovascular Research Foundation’s Transcatheter Cardiovascular Therapeutics (TCT) ...

Oct. 1, 2013 – The Cardiovascular Research Foundation (CRF) announced the late breaking trials and first report ...
The year 2013 has brought us several important clinical trials that have changed the way interventional cardiologists ...

June 14, 2013 — Preliminary results from the ADVISE (Adenosine Vasodilator Independent Stenosis Evaluation) II trial ...

 September 12, 2014
September 12, 2014