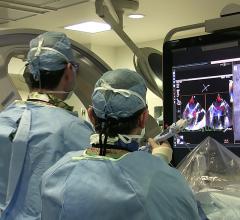
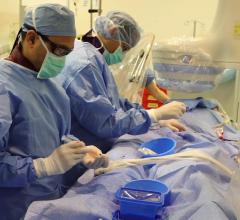
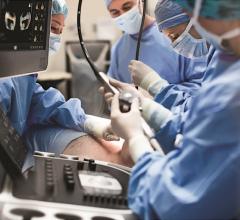
Dr. Neil Moat, MBBS, chief medical officer of Abbott's structural heart business, was a cardiac surgeon specializing in the mitral valve prior to taking on his current role where he oversees Abbott's ...
Hybrid OR
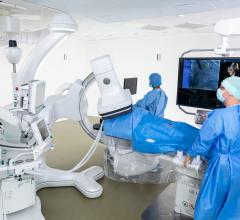
This channel includes news and new technology innovations connected with hybrid operating rooms, also referred to as hybrid ORs, hybrid cath labs or hybid interventional suites. These rooms combine catheterization lab and OR technologies and requirements fro both open surgical and transcatherer procedures.
Dr. Neil Moat, MBBS, chief medical officer of Abbott's structural heart business, was a cardiac surgeon specializing in ...
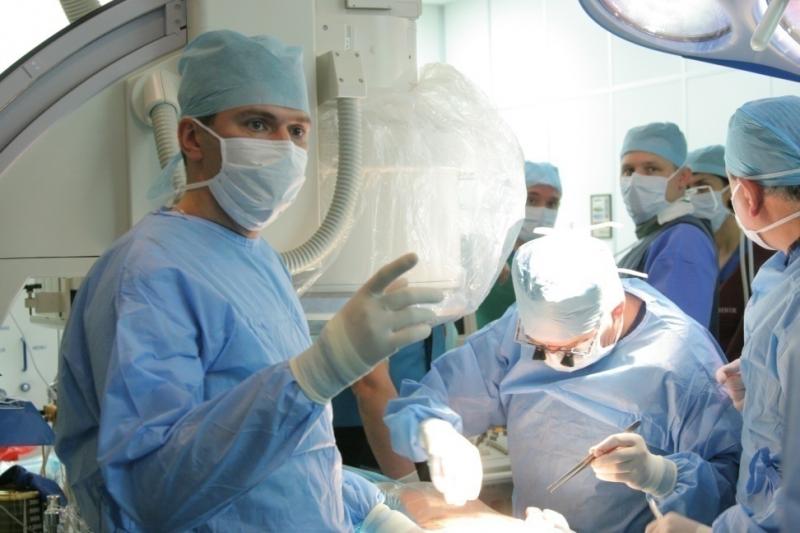
The resounding success of transcatheter aortic valve replacement (TAVR) has led the creation of hundreds of structural ...
As a medical technology journalist, a little more than a decade ago I found myself sitting in those late afternoon ...
December 3, 2020 — GE Healthcare is introducing a new version of its robotic driven angiography system for image guided ...
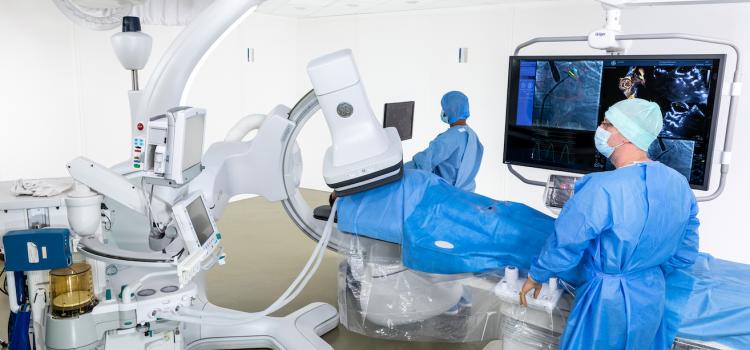
October 2, 2020 — The new cardiac hybrid operating room (OR) at London Health Sciences Centre (LHSC) in London, Ontario ...
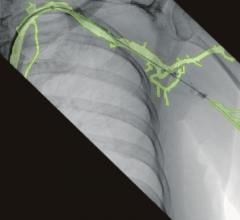
August 18, 2020 — Ziehm Imaging announces the acquisition of Therenva, a French-based developer of planning and imaging ...
Richard Botto, CVT, RCSA, chief cardiovascular technologist, division of cardiology, cardiac cath lab, offers an ...
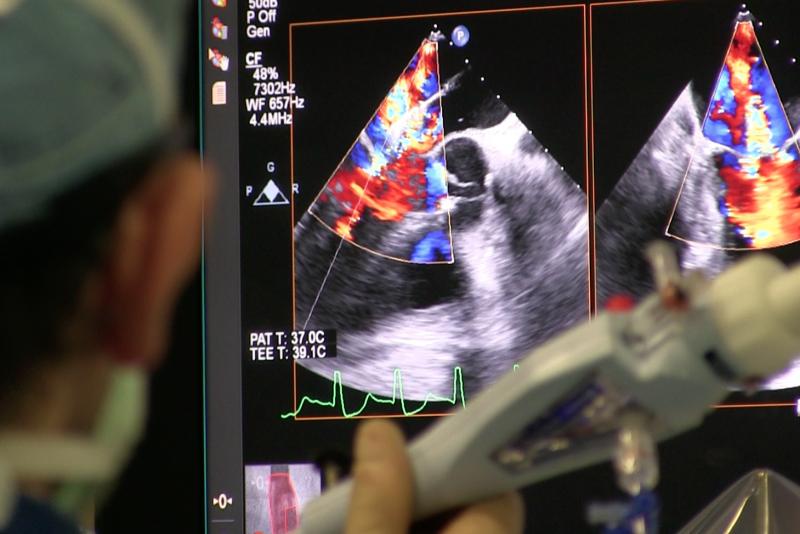
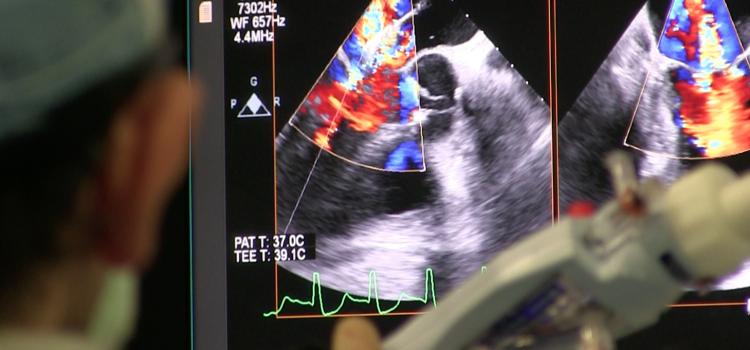
June 4, 2020 — Intra-operative transesophageal echocardiography (TEE) is a versatile diagnostic and monitoring tool used ...
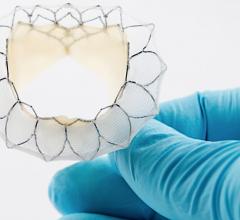
May 29, 2020 — Neovasc Inc., developer of a minimally invasive transcatheter mitral valve replacement (TMVR) ...
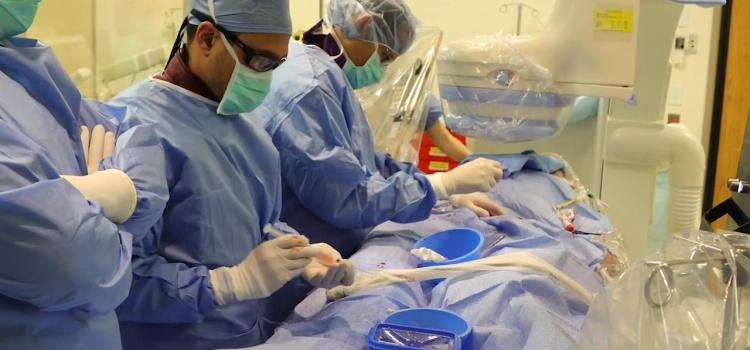
This is a walk through of the primary structural heart hybrid cath lab at Henry Ford Hospital in Detroit, Mich. It is ...
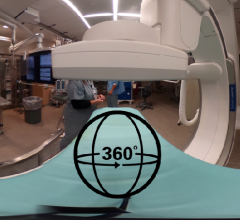
This is a 360 degree image showing the main hybrid cath lab at Henry Ford Hospital in Detroit, which is used for most of ...
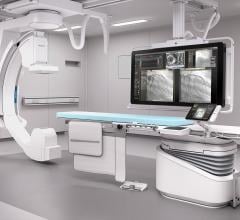
March 3, 2017 — Philips recently announced the global launch of Azurion, its next-generation image-guided therapy platfo ...
In the current era of healthcare reform and the push toward evidence-based medicine to both lower costs and improve ...
November 18, 2016 — Mitralign Inc. announced the successful compassionate use treatment of a patient suffering from tric ...
A discussion with Juan Granada, M.D., about transcatheter mitral valve advancements and device challenges at the Transca ...

 October 15, 2021
October 15, 2021