Sept. 16, 2025 — Elutia Inc., a pioneer in drug-eluting biomatrix technologies, has published clinical and preclinical data supporting the clinical utility of a biologic envelope that secures cardiac ...
Implantable Cardioverter Defibrillator (ICD)
This channel includes news and new technology innovations for implantable cardioverter defibrillators (ICD) used to treat tachycardia arrhythmias and heart failure. This includes cardiac resynchronization therapy defibrillators (CRT-D).
Jan. 12, 202 — Abbott and AtaCor Medical have announced a collaboration to advance a next-generation investigational ...
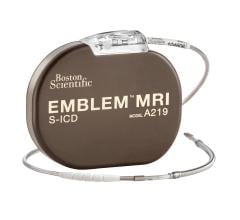
Sept. 16, 2025 — Elutia Inc., a pioneer in drug-eluting biomatrix technologies, has published clinical and preclinical ...

As healthcare systems continue to shift toward value-based care and proactive disease management, remote monitoring has ...
Sept. 2, 2024 – Medtronic recently shared long-term results from the global Extravascular Implantable Cardioverter ...
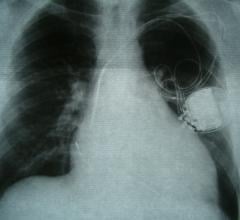
June 17, 2024 — Elutia Inc., a pioneer in drug-eluting biomatrix products, today announced that its Antibiotic-Eluting ...
May 18, 2024 — Boston Scientific Corporation today announced positive six-month results from the ongoing pivotal MODULAR ...
February 16, 2024 — AMO Pharma Limited, a privately held clinical-stage specialty biopharmaceutical company focusing on ...
February 14, 2024 — The American College of Cardiology’s newest registry offers data-driven insights on cardiac ...
February 12, 2024 — BIOTRONIK, a leader in implantable medical device technology, announced today they will solely ...
January 11, 2024 — It is with great sadness that the Diagnostic and Interventional Cardiology (DAIC) team has learned of ...
November 22, 2023 — The first clinical trial to challenge the routine implantation of a defibrillator in myocardial ...
October 23, 2023 — Medtronic plc, a global leader in healthcare technology, has received U.S. Food and Drug ...
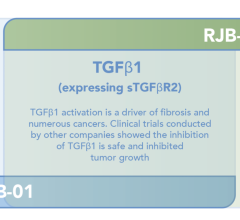
October 9, 2023 — Rejuvenate Bio announced new preclinical efficacy data for RJB-0402, a gene therapy targeting key ...
August 31, 2023 — The European Society of Cardiology (ESC) Guidelines on cardiomyopathies are published online in Europe ...
August 28, 2023 — Is the routine implantation of an implantable cardioverter defibrillator (ICD) in myocardial ...





 January 13, 2026
January 13, 2026