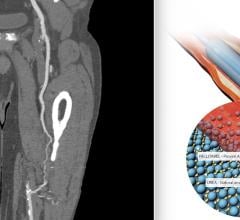
July 12, 2022 — Biotronik announced the presentation of two studies on the performance of its drug-coated balloon ...
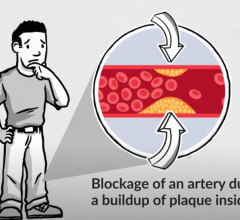
Peripheral Artery Disease (PAD)
This channel includes news, interventions, and new technology innovations for peripheral artery diease, PAD and critical limb ischemia.
June 13, 2022 — Royal Philips announced the latest results from the Tack Optimized Balloon Angioplasty (TOBA) II below ...
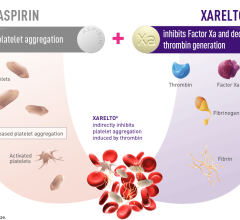
May 31, 2022 — Findings from the XARELTO (rivaroxaban) Phase 3 COMPASS Long-Term Open Label Extension (LTOLE) study and ...
May 20, 2022 — New long-term data from the Safety Assessment of Femoropopliteal Endovascular Treatment With PAclitaxel ...
May 19, 2022 — One year outcomes from the Disrupt PAD III Trial comparing intravascular lithotripsy (IVL) with a drug ...
April 12, 2022 — Transit Scientific announced the FDA clearance of its XO Cross Support Catheter Platform to include ...
March 22, 2022 – With 60 years of experience, The Cardiovascular Care Group is nationally recognized for vascular ...
March 9, 2022 — Medtronic is recalling (correcting) the TurboHawk Plus Directional Atherectomy System due to design ...
February 21, 2022 – A research group the Department of Cardiovascular Medicine, Osaka City University Graduate School of ...
February 15, 2022 – Alkem Laboratories Limited (Alkem), an Indian multinational pharmaceutical company, has signed a ...
January, 24, 2022 — Medtronic Inc. is recalling its HawkOne Directional Atherectomy System product due to the risk of ...
January 17, 2022 – The Vascular Care Group (TVCG) announced Stephen J. Hoenig M.D., successfully completed a ...
November 10, 2021 — Abbott released new global market research from its Beyond Intervention initiative, the company’s ...
November 9 2021 — A new large-scale, real-world analysis of Centers for Medicare and Medicaid Services (CMS) outcomes ...
October 20, 2021 — Philips, a global leader in health technology and intravascular ultrasound (IVUS) solutions ...


 July 12, 2022
July 12, 2022