August 28, 2019 — The U.S. Food and Drug Administration (FDA) released an updated MedWatch Alert this month on the ...
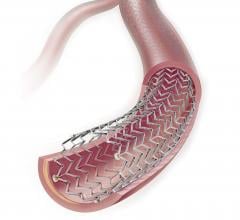
Stents Peripheral
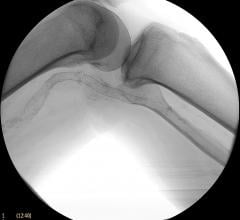
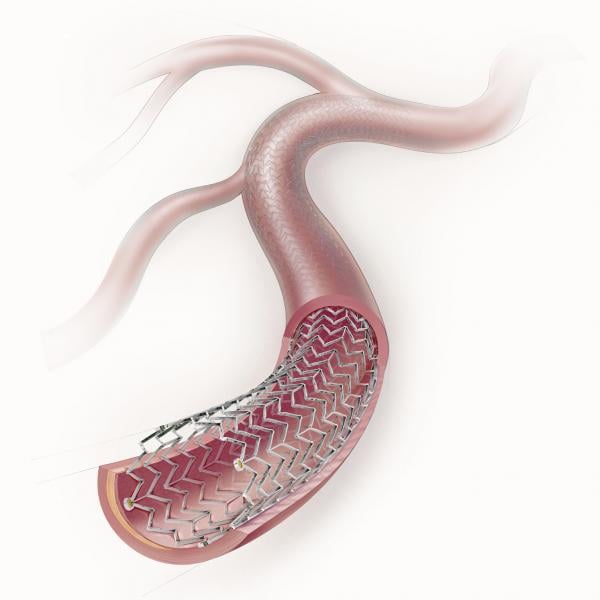
Peripheral stents are used to open narrow and hardening arteries that supply blood to the legs and feet.
August 1, 2019 — Less-invasive procedures to open severely clogged leg arteries were as good at helping people survive ...
April 15, 2019 – Intact Vascular Inc. received U.S. Food and Drug Administration (FDA) market clearance for the Tack ...

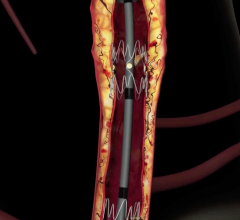
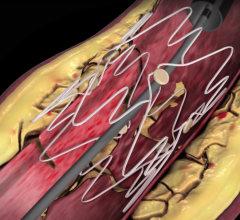
In recent years, there has been a lot of focus by vendors on developing better stenting technologies to treat peripheral ...
The anti-proliferative drug paclitaxel has been used as a coating on coronary stents to prevent restenosis since 2003 ...
January 17, 2019 — The U.S. Food and Drug Administration (FDA) issued a letter Jan. 17, 2019, to healthcare providers ...
Professor Ian Meredith, MBBS, Ph.D., global chief medical officer and executive vice president, Boston Scientific ...
October 5, 2018 — Veryan Medical Ltd has received Premarket Approval (PMA) for the BioMimics 3D Vascular Stent System ...
May 15, 2018 — The Society for Cardiovascular Angiography and Interventions (SCAI) has released new guidelines to ...
April 27, 2018 — Intact Vascular Inc. recently closed a Series C financing totaling $20 million. This financing is ...
February 1, 2018 – Thomas Zeller (Bad Krozingen, Germany) presented the 12-month results from Veryan Medical’s MIMICS-2 ...

September 14, 2017 — Here are quick summaries for all the key late-breaking vascular and endovascular clinical trials ...
July 26, 2017 — Intact Vascular Inc. recently announced that its Tack Optimized Balloon Angioplasty II Below the Knee ...
July 17, 2017 — LimFlow SA announced enrollment of the first patient in the U.S. feasibility study of the LimFlow ...
July 14, 2017 — Intact Vascular Inc. announced the U.S. Food and Drug Administration (FDA) approved an Investigational ...


 August 28, 2019
August 28, 2019