December 1, 2023 — Researchers have succeeded in restoring lost brain function in mouse models of stroke using small ...
Stroke
This channel includes news and new technology innovations for stroke. It includes both diagnosis and treatment of stroke, stroke imaging, pharmaceuticals and interventional stroke technologies. Stroke comes in two forms, which have different therapies.
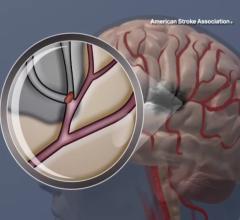
1. Ischemic stoke is a blockage of an artery in the brain, preventing blood flow and is offen referred to as a "brain attack" because it is a similar casue as a heart attack. This type of stroke is often treated with anti-coagulants, including use of tissue plasminogen activator (tPA). Interventional mechanical thrombectomy can also be used to remove the clot.
2. Hemorrhagic stroke is caused when there is bleeding due to a ruptured blood vessel in the brain caused by a brain aneurysm burst or a weakened blood vessels. These strokes are less common, but exact diagnosis is important, because use of tPA in these patients can have catastrophic consequences. Treatments include interventional embolization and surgical clipping of target vessels.
November 30, 2023 — Johnson & Johnson MedTech1 today announced the completion of the acquisition2 of Laminar, Inc., a ...
November 29, 2023 — InspireMD, Inc., developer of the CGuard Embolic Prevention Stent System (EPS) for the prevention of ...
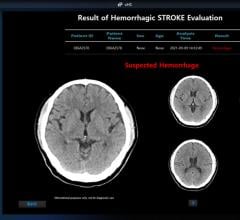
November 21, 2023 — Heuron, a medical AI (artificial intelligence) imaging software solution company, under the ...
November 15, 2023 — Researchers at the University of Leicester have discovered the mechanism by which cholesterol in our ...
November 14, 2023 — Deepak Srivastava, MD, president of Gladstone Institutes and a renowned cardiovascular researcher ...
November 14, 2023 — Eko Health, Inc., a leader in AI technology for heart and lung disease detection, today unveiled new ...
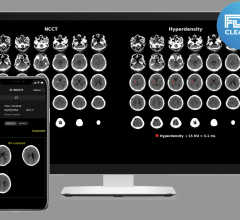
November 9, 2023 — RapidAI, a global leader in developing artificial intelligence (AI) and technology workflow solutions ...
November 8, 2023 — The combination of persistent tobacco use and prediabetes (higher than normal blood sugar levels that ...
November 3, 2023 — In the U.S., someone has a stroke every 40 seconds and dies from it every three minutes and 14 ...
November 2, 2023 — Contego Medical Inc. announced the presentation of late-breaking clinical results from the ...
October 23, 2023 — Investigators from the Smidt Heart Institute at Cedars-Sinai found that an artificial intelligence (A ...
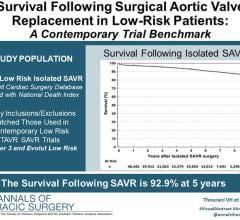
October 18, 2023 — A study published in The Annals of Thoracic Surgery demonstrates outstanding long-term survival ...
October 17, 2023 — Patients with a previous or current cancer diagnosis are more likely to have a stroke than the ...

 December 01, 2023
December 01, 2023