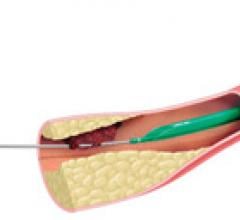
April 2, 2012 — Interventional radiology treatments re-establish blood flow in people with chronic deep vein thrombosis ...
Thrombectomy Devices
This channel includes news and new technology innovations for catheter-based thrombectomy systems used to remove blood clots from vessels in the body. Thrombus removal a decade ago was a standard of care for acute coronary revascularization in ST-elevation myocardial infarction (STEMI). But, its use declined rapidly after several large trials showed no benefit. However today, thrombectomy is seeing increasing usage in new therapeutic areas to treat acute stoke, pulmonary embolism (PE), deep vein thrombosis (DVT) and venous thromboembolism (VTE). Thrombectomy is also referred to as embolectomy.
The types of thrombectomy systems include:
● Ultrasound-assisted thrombolysis – Catheter-directed, high-frequency ultrasound helps thrombolytic agents penetrate clots to speed the action of fibrinolytic pharmacological therapy.
● Rheolytic embolectomy – These devices inject pressurized saline through the catheter's distal tip and the macerated thrombus is aspirated through a catheter port.
● Rotational embolectomy – A rotating device at the catheter tip is used to fragment the clot and fragments are aspirated by the catheter.
● Aspiration thrombectomy – This includes manual clot aspiration or use of dedicated aspiration catheter devices that basically vacuum the clot out of the vessel.
● Thrombus fragmentation – Thrombus can be mechanically disrupted by manually rotating a pigtail catheter or using balloon angioplasty, but this causes small fragments of emboli to flow distally. Dedicated devices also are available.

March 27, 2012 – Researchers found the antiplatelet drug abciximab significantly decreased damage to the heart muscle in ...
January 23, 2012 — Pregnant women who develop dangerous blood clots in the leg often forgo the most effective treatment ...
January 20, 2012 - Robert J. Kennedy, M.D., interventional radiologist at Holmes Regional Medical Center in Melbourne ...
September 8, 2011 – Concentric Medical announced completion of enrollment of the TREVO Study. The TREVO Study ...
September 6, 2011 — Stryker Corp. has signed a definitive agreement to acquire Concentric Medical for $135 million in an ...
July 26, 2011 — Concentric Medical Inc. has launched the DAC 070 Catheter, the fourth and largest diameter addition to ...
July 21, 2011 – Concentric Medical Inc. announced the launch of the expanded family of Merci Retrievers in Japan for the ...
Coronary revascularization does not always lead to coronary reperfusion. When readily available, percutaneous coronary ...
May 23, 2011 - Doctors in Germany are among the first to treat ischemic stroke patients outside the United States with ...
April 4, 2011 – Doctors are encouraged to consider therapies in addition to blood thinners to treat certain ...
March 31, 2011 – Deep vein thrombosis, or DVT is more than just a one-time complication from a long plane ride ...
March 25, 2011 – A new, next-generation aspiration catheter has been introduced in the United States. The Fetch 2 ...
March 7, 2011 – Each year between 100,000 and 180,000 Americans die as the result of pulmonary embolism, a ...
February 9, 2011 – The first patients have been enrolled in a trial investigating the safety and efficacy of a ...

 April 02, 2012
April 02, 2012