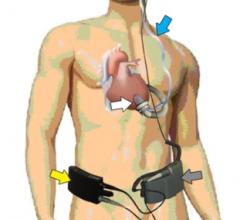
March 13, 2013 — CircuLite Inc. announced it has received conditional approval from the U.S. Food and Drug ...
Ventricular Assist Devices (VAD)
This channel includes news and new technology innovations for ventricular assist devices (VAD). VADs are a type of mechanical hemodynamic support device that helps increase blood flow in people who have ventricles that are not work properly due to heart failure, cardiogenic shock, cardiomyopathy or myocardial infarction. Most often these devices support the left ventricle, so they are often referred to as left ventricular assist devices (LVAD). VADS come in two types, surgically implanted, usually as a bridge to heart transplant, and percutanenous catheter-based pumps used for temporary hemodynamic support. Examples of temporary percutaneous pumps include the Impella and TandemHeart devices.
My job as editor of DAIC is to help readers keep tabs on the latest in cardiovascular technology and trends. With ...
December 19, 2012 — CardiacAssist Inc. announced it has received investigational device exemption (IDE) approval from ...
December 17, 2012 — Abiomed Inc. announced the U.S. Food and Drug Administration's (FDA) Circulatory System Devices ...
Two key news items in September made me think about the future direction of hemodynamic support, and that intra-aortic ...

Two key news items in September made me think about the future direction of hemodynamic support, and that intra-aortic ...
December 6, 2012 — The U.S. Food and Drug Administration (FDA) granted premarket approval (PMA) for the HeartWare ...
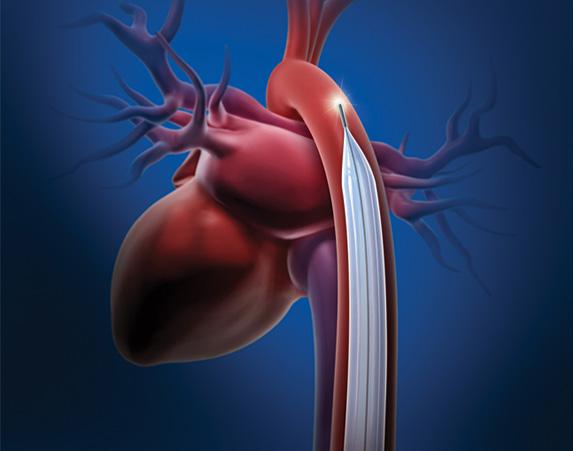
November 8, 2012 — A U.S. Food and Drug Administration (FDA) advisory committee is meeting Dec. 5 to discuss how to ...
October 3, 2012 — The National Institutes of Health (NIH)/National Cancer Institute (NCI) has awarded a five-year ...
October 1, 2012 — CircuLite Inc. announced that the latest data from patients enrolled in the company’s CE mark trial of ...
September 18, 2012 — Abiomed Inc. announced it received confirmation from the American Medical Association (AMA) of ...
CircuLite Inc. announced that it has received CE Marking approval for the Synergy Circulatory Support System, a micro ...
September 11, 2012 -- Abiomed Inc. announced it has received 510(k) clearance from the U.S. Food and Drug Administration ...

September 11, 2012 — Primary results from an ongoing trial by Maquet Cardiovascular using intra-aortic balloon pumps ...
August 17, 2012 — Jarvik Heart Inc. announced U.S. Food and Drug Administration (FDA) approval of its pivotal trial for ...


 March 13, 2013
March 13, 2013