August 14, 2019 — Ancora Heart Inc. announced the first patient was enrolled in the CorCinch EU study, a European multi-center clinical evaluation of the AccuCinch Ventricular Repair System as a treatment for patients with reduced ejection fraction systolic heart failure (HFrEF).
The first patient was enrolled at Homolka Hospital in Prague, Czech Republic by Prof. Petr Neužil, M.D., CSc., FESC, head of the department of cardiology at Homolka Hospital and principal investigator of the study. The AccuCinch procedure was completed by co-investigator Vivek Reddy, M.D., director of cardiac arrhythmia services at The Mount Sinai Hospital in New York, along with Neužil.
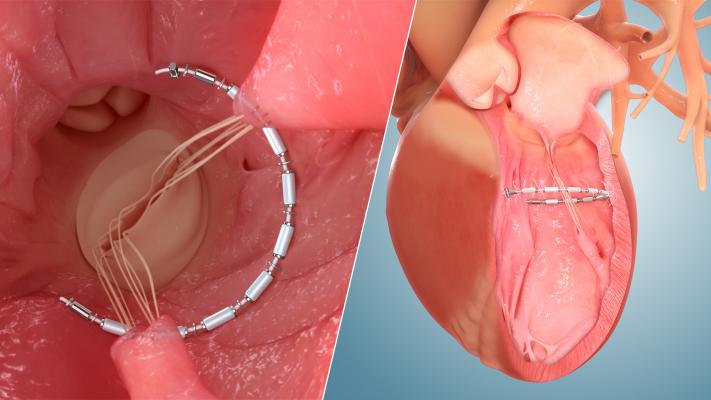
“The transcatheter AccuCinch therapy is unique in its ventricular approach, which is intended to improve heart function by repairing the left ventricle for patients with systolic heart failure regardless of its origin,” said Neužil. “We are pleased to participate in this study because the AccuCinch has the potential to address the shortcomings of current medical, surgical and transcatheter treatments and restore quality of life and longevity for this large patient population.”
This is the second recently initiated Ancora study evaluating the AccuCinch system focused specifically on heart failure and the enlarged left ventricle. The study will enroll up to 132 patients from heart centers across Europe.
The transcatheter AccuCinch therapy is designed to complement and enhance the existing care cardiologists provide to further manage symptoms and slow, or stop, the progression of heart failure. For some patients, AccuCinch may have the potential to reverse the enlargement of the left ventricle. For patients where heart failure has progressed beyond the ability for medications and pacemakers to manage symptoms, non-surgical percutaneous device therapy with AccuCinch may provide an effective treatment option. The AccuCinch system is designed to directly repair the left ventricle of the heart, thereby addressing the fundamental issue in the progression of systolic heart failure.
An estimated 25 million adults worldwide live with heart failure, a condition in which the heart’s muscles slowly weaken and lose their ability to pump enough oxygen-rich blood to the body.1 Heart failure patients suffer from debilitating symptoms including persistent exhaustion, trouble breathing, confusion and loss of memory. About half of heart failure patients have an enlarged left ventricle, the main pumping chamber of the heart, which causes more stress on the heart and leads to reduced pumping efficiency. Current heart failure treatments only partially address the enlarged left ventricle and up to 50 percent of people who develop heart failure die within five years of diagnosis.1
For more information: www.ancoraheart.com
References


 January 05, 2026
January 05, 2026 









