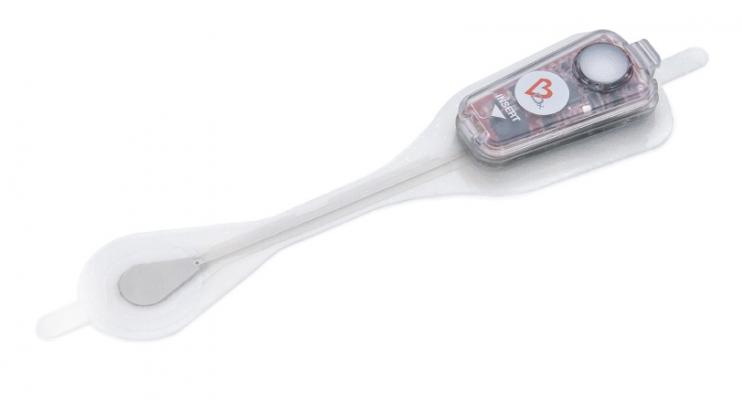
June 14, 2019 — Bardy Diagnostics Inc. announced that its Carnation Ambulatory Monitor (CAM) was recognized with the “Best New Diagnostic Technology” award in the 2019 MedTech Breakthrough Awards Program. MedTech Breakthrough is an independent organization that recognizes the top companies and solutions in the global health and medical technology market. BardyDx earned the distinction for its P-wave centric ambulatory cardiac patch monitoring and arrhythmia detection technology.
Reliably detecting and recording the P-wave, a small amplitude signal of the electrocardiogram (ECG) originating in the atrium, is essential to accurate arrhythmia diagnosis and the determination of appropriate medical or procedural intervention.
The MedTech Breakthrough Awards program is the premier awards and recognition platform founded to recognize the medical and health technology innovators, leaders and visionaries from around the world. The program evaluates and recognizes breakthrough solutions and companies in a range of categories, including Medical Devices, mHealth, Robotics, TeleHealth, Patient Engagement, Electronic Health Records (EHR), Healthcare Security, Medical Data and more. This year's program attracted more than 3,500 nominations from over 15 different countries throughout the world that were evaluated by an independent panel of experts within the medtech industry, with the winning products and companies selected based on a variety of considerations, including innovation, design and user experience.
"BardyDx's non-invasive P-wave centric CAM patch is disrupting decades of old practice and technology for cardiac diagnostics and monitoring," said James Johnson, managing director, MedTech Breakthrough. "CAM represents a true digital health breakthrough and we are thrilled to recognize the company with a 2019 MedTech Breakthrough Award in recognition of their outstanding success and innovation in the digital health space."
Last month, BardyDx was named both the winner of the 2019 Frost & Sullivan Award for Technology Innovation in Remote Cardiac Monitoring and the winner of the 2019 GeekWire Award in the Hardware of the Year category. Also, BardyDx was recently named the winner of the Impact Pediatric Health Competition hosted by the nation's leading pediatric healthcare institutions at SXSW 2019 for the CAM's pediatric-friendly design and potential to address significant unmet needs in pediatric healthcare. In addition, BardyDx was also selected as the winner of the 2018 Fierce Innovation Life Sciences Award for Medical Device Innovation from the leading industry publisher of FierceBiotech & FiercePharma.
The full list of the 2019 MedTech Breakthrough Award winners can be found here.
For more information: www.bardydx.com


 November 12, 2025
November 12, 2025 









