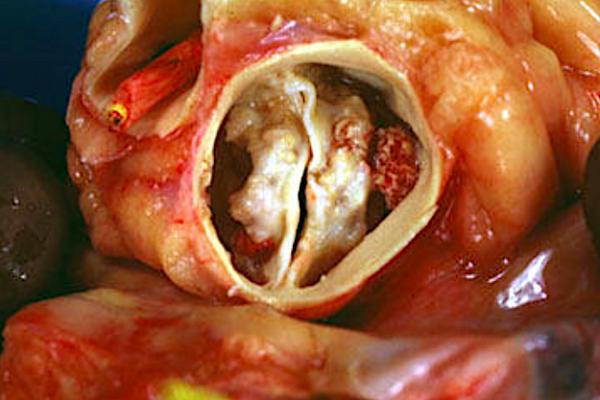
February 4, 2021 — Transcatheter aortic valve replacement (TAVR) has historically only been used with caution in the patients with bicuspid valve valves, but recent studies may soon open the door to wider use of TAVR in this population.
“Physicians have previously been cautious about using TAVR in patients with bicuspid valve stenosis due to a number of factors," explained Ashley Young, senior medical devices analyst at GlobalData healthcare market research. "Perhaps most importantly, the long-term durability of TAVR valves has yet to be thoroughly proven in a robust clinical trial setting. This is of utmost importance to patients with bicuspid aortic valve anatomy, who typically present with stenosis at a younger age and depend on long-term durability of replacement valves.”
GlobalData predicts that up to 53% of adult patients with diagnosed severe aortic stenosis have bicuspid valve anatomy, representing a huge potential for market expansion if TAVR devices are able to extend into this population. With the recent update to the precautionary labels of both the Edwards Lifesciences and Medtronic TAVR valves, this seems like a possibility for the near future.
“Once physicians become more comfortable with the recent clinical data supporting the use of TAVR devices in patients with bicuspid valve anatomy, GlobalData predicts that TAVR usage in this population will grow at a double-digit CAGR, mimicking the success of the procedure in the tricuspid valve population,” Young said.
A late-breaking study at the 2020 American College of Cardiology (ACC) meeting was the first to prospectively examine TAVR’s safety for treating severe aortic stenosis in relatively young, healthy patients—in whom open-heart surgery would pose a low risk—who have a bicuspid valve. It also is one of the first studies involving such patients in which doctors used a newer self-expanding artificial valve. The study prospectively tracked 150 patients who underwent TAVR at 25 medical centers in the U.S. and found TAVR is a safe alternative to surgery in bicuspid patients.
“This clearly has clinical implications with patients with bicuspid valves who want TAVR," said Basel Ramlawi, M.D., cardiothoracic surgeon at Lankenau Medical Center and the study’s co-principal investigator. "TAVR with a self-expanding prosthesis is a very viable and safe procedure in low-risk bicuspid patients and achieved excellent early results. Though additional follow-up is necessary to determine long-term outcomes, early results suggest this procedure can be performed successfully in low-risk individuals with a good outcome.”
Aortic stenosis is one of the most common valvular heart diseases, with the moderate to severe form of the disease affecting nearly 2 million people in the U.S. alone. While the majority of people have tricuspid aortic valve anatomy with three valve leaflets, a portion of the population has bicuspid aortic valve anatomy with only two leaflets. This variation of anatomy only affects between 2-5 percent of people, but the anatomy makes makes it more likely to develop calcification of the valve that leads to aortic stenosis.
GlobalData said the use of TAVR is rising at a compound annual growth rate (CAGR) of 1% in most countries.
For more information: www.globaldata.com
Related Bicuspid TAVR Content:
VIDEO: TAVR Performs Well in Bicuspid Aortic Valve Patients — interview with Basel Ramlawi, M.D.
Canada Clears Medtronic Evolut TAVR for Both Bicuspid Valves and for All Low Risk Patients
VIDEO: Explaining Bicupid Aortic Valves


 November 14, 2025
November 14, 2025 









