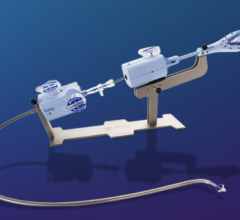
October 14, 2015 — Boston Scientific Corp. announced it has closed on an additional round of financing with MValve Technologies Ltd., developer of a percutaneous mitral valve replacement system designed to work with the Boston Scientific Lotus Valve. Boston Scientific has provided the company with funding since 2012 and has an exclusive option to acquire MValve.
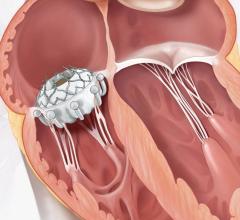
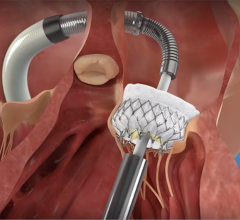
MValve Technologies plans to utilize the new financing, in part, to fund a first-in-human clinical trial for the MValve docking system for transcatheter mitral valve replacement (TMVR) in patients with mitral regurgitation. The approach, in which the Boston Scientific Lotus Valve is deployed inside the MValve docking system, is designed to enable the treatment of mitral regurgitation in a broad range of patients, and to improve long-term clinical outcomes in this patient population. Both the dock and valve can be repositioned and recaptured, enabling precise valve placement and physician confidence prior to releasing.
Mitral regurgitation is the most common type of heart valve disorder and occurs when the mitral valve does not close properly, causing an abnormal reversal of blood to flow from the left ventricle into the left atrium.
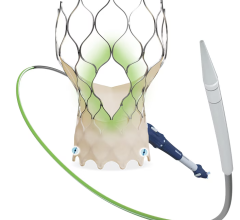
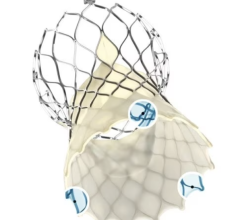
The Lotus Aortic Valve System is a differentiated second-generation valve replacement technology, consisting of a pre-loaded, stent-mounted tissue valve prosthesis and catheter delivery system for guidance and percutaneous placement of the valve. The low-profile delivery system is designed to enable predictable and precise placement associated with early valve function, as well as bi-directional atraumatic repositioning and retrieval at any time prior to release of the aortic valve implant. The device also features a unique Adaptive Seal designed to minimize the incidence of paravalvular regurgitation.
In the United States, the Lotus Valve System is an investigational device and not available for sale. It is CE marked in the European Union.
For more information: www.bostonscientific.com


 July 08, 2024
July 08, 2024