December 21, 2012 — Covidien announced the five-year results of the ClosureFast long-term European multicenter study in patients with chronic venous insufficiency (CVI). This study evaluates the five-year outcomes of a minimally invasive endovascular therapy for treatment of CVI.
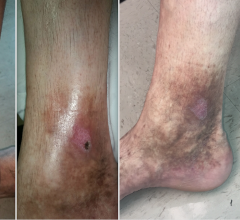
The ClosureFast study was conducted prospectively at eight centers in Europe. The Venefit procedure (using the ClosureFast radiofrequency ablation catheter) was used to treat 295 lower limbs in 225 patients diagnosed with superficial venous reflux, the underlying cause of varicose veins and CVI. This progressive medical condition affects the veins in the leg and compromises their ability to carry blood back to the heart. If left untreated, varicose veins can advance to CVI, which, in severe cases, may result in lower leg pain, skin damage and leg ulcerations.
"The five-year final analysis from the ClosureFast study shows sustained treatment success in anatomical and clinical categories and supports its leading position in endovenous vein treatment," said Thomas Proebstle, M.D., Universitaets Hautklinik Heidelberg, Germany, who recently delivered the study results at the American College of Phlebology Annual Congress.
ClosureFast Study Design and Five-Year Results
This prospective, multicenter study enrolled 225 patients and treated 295 limbs at eight centers in Europe from April 2006 to June 2007. The study treated patients diagnosed with superficial venous reflux with the minimally invasive Venefit procedure using the ClosureFast radiofrequency ablation catheter.
Patients were evaluated following the procedure by duplex ultrasound imaging and clinical examination at three days, three months, six months and in yearly intervals up to five years. Of the 295 treated limbs, 233 (79 percent) were available for examination at five years. According to Kaplan-Meier analysis, at five-year follow-up, full occlusion of the treated vein was observed in 92 percent of patients (vs. 98 percent at one year). The vast majority (95 percent) of patients were free of pathological venous reflux (vs. 99 percent at one year). This demonstrates durable results of the Venefit procedure out to five years.
The average venous clinical severity score (a multifactorial score measuring disease severity and quality of life) improved from 3.9 ± 2.1 before treatment to 1.3 ± 1.7 at 5 years. In addition, only 14 percent of limbs were CEAP (disease severity classification) class 3 or higher at 5 years, while, at baseline, 48 percent of limbs were in the same category.
“This pivotal study provides substantial long-term data on relevant endpoints to help clinicians decide the appropriate treatment for their patients, thus defining Covidien’s commitment to a strong foundation of clinical research,” said Mark A. Turco, M.D., chief medical officer, vascular therapies, Covidien.
For more information: www.covidien.com


 September 10, 2025
September 10, 2025