October 26, 2017 — Medical professionals representing hospital systems from across the United States are expected to attend the first National Cardiogenic Shock Initiative (NCSI) meeting Oct. 29 in Denver. The gathering will help kick off Transcatheter Cardiovascular Therapeutics (TCT) 2017, an annual educational meeting specializing in interventional cardiovascular medicine.
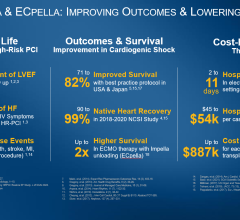
The National Cardiogenic Shock Initiative is a natural evolution of the successful Detroit Cardiogenic Shock Initiative. Doctors across southeast Michigan increased cardiogenic shock survival rates from 51 percent to 76 percent by organizing and following a protocol using a straw-sized heart pump.
The pioneering cardiologist who led the Detroit effort, William W. O’Neill, M.D., medical director of the Center for Structural Heart Disease at Henry Ford Hospital, is leading the NCSI effort and the meeting in Denver.
“We saw such success in Detroit, and we would like to share this life-saving knowledge with others,” said O’Neill, who presented an analysis of hemodynamic support to a standing-room-only crowd at the American College of Cardiology’s Scientific Sessions in Washington, D.C. earlier this year.
The devastating heart attack complication cardiogenic shock affects approximately 5-8 percent of heart attack patients in the United States annually. In these patients, the pump function of the heart is severely depressed, causing low blood pressure and vital organs to be deprived of sufficient blood supply. Despite contemporary treatments, an average of about 50 percent of patients experiencing the condition die.
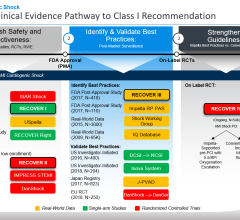
But heart attack survival rates increased dramatically in cardiogenic shock patients who were provided rapid hemodynamic support before treating the cause of a heart attack. The analysis looked at the use of the Impella, a straw-sized pump approved by the U.S. Food and Drug Administration (FDA) in 2008 and specifically for treatment of cardiogenic shock in 2016. The pump is inserted through a catheter in the groin and into the heart to keep blood pumping throughout the body. Doctors use it while they’re treating the cause of a heart attack, either inserting a stent, removing a clot or taking other necessary action while the tiny pump supports circulation.
The basis for the protocol used retrospective data collected by the pump’s manufacturer, Massachusetts-based Abiomed, in 15,529 patients – 72 percent men and an average age of 63 – treated between 2009 and 2017.
The investigators noticed that survival rates in patients treated with the Impella pump fluctuated wildly by hospital, O’Neill said. A third of hospitals experienced a 25 percent survival rate; a third, 50 percent survival; and a third, 75 percent.
“There was a huge variation in outcomes between the centers, between the lowest performing and highest performing centers,” said O’Neill. “It looked as though putting the Impella in soon, before you do anything else, is connected to survival.”
Inserting the pump first ensures the patient’s other vital organs are supported, buying time for cardiologists to open the blocked arteries to restore natural blood flow. The hospitals with high survival rates also used less inotropes and catheters to monitor heart pressure, O’Neill added.
To test the best practices, O’Neill organized five hospital systems in southeast Michigan to follow a specific protocol in acute myocardial infarction patients who showed signs of cardiogenic shock between July 2016 and April 2017. Of the 41 patients supported with the Detroit Cardiogenic Shock Initiative protocol, 31 (76 percent) survived
“Some of these breakthroughs are so straightforward, you wonder, ‘Why didn’t we think of it before?’” O’Neill said.
Participants with Henry Ford Health System in the Detroit Cardiogenic Shock Initiative include Beaumont Hospital, Detroit Medical Center, Ascension’s St. John and Providence hospitals and Saint Joseph Mercy Health System.
Key to the protocol is medical personnel recognizing cardiogenic shock in patients as soon as possible, O’Neill added.
“If they think the patient needs medication to raise the blood pressure, that’s when they should be thinking, ‘Get them someplace where they can get a pump implanted,’” O’Neill said.
For more information: www.henryford.com/cardiogenicshock
Related Cardiogenic Shock Content
Improving Cardiogenic Shock Survival With New Protocols
VIDEO: Overview of the National Cardiogenic Shock Initiative
VIDEO: Cardiogenic Shock Supported With Impella Shows Good Outcomes
VIDEO: Demonstration of the Impella Percutaneous Hemodynamic Support Device


 April 28, 2023
April 28, 2023