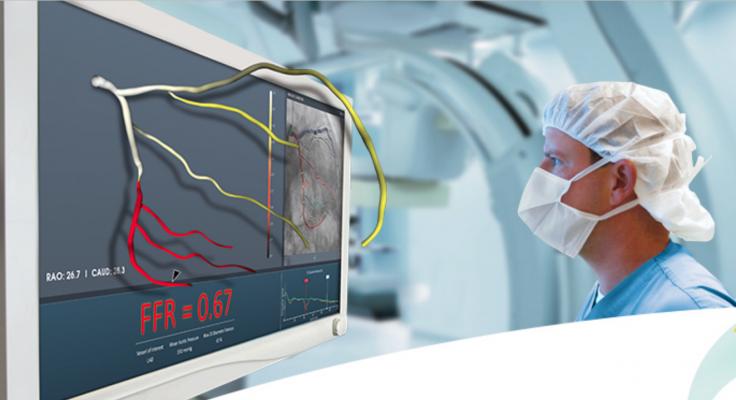
The late-breaking FAST-FFR Trial demonstrated that the sensitivity and specificity of the CathWorks FFRangio angiography imaging derived fractional flow reserve (FFR-angio) technology matched the performance of wire-based FFR measurements.
October 4, 2018 — The late-breaking FAST-FFR Trial demonstrated that the sensitivity and specificity of the CathWorks FFRangio angiography imaging derived fractional flow reserve (FFR-angio) technology matched the performance of wire-based FFR measurements. The data were presented at the 2018 Transcatheter Cardiovascular Therapeutics (TCT) annual meeting.
The sensitivity and specificity were 93.5% and 91.2%, both of which exceeded the trial’s pre-specified performance goals. The diagnostic accuracy was 92% overall, and remained high when considering only FFR values in the critical zone between 0.75 and 0.85. As a result, the trial concluded that the CathWorks System has the promise to substantially increase physiologic coronary lesion assessment in the cath lab, potentially leading to improved patient outcomes. The results were simultaneously published in Circulation. [1]
The CathWorks FFRangio System is a non-invasive FFR platform that quickly and precisely delivers objective multi-vessel physiologic measurements to cost-effectively optimize and confirm intra-procedural percutaneous coronary intervention (PCI) therapy decisions. It is designed to deliver the objective FFR guidance needed to optimize PCI therapy decisions for every patient.
Stephan Achenbach, M.D., FSCCT is chairman of cardiology and professor of medicine at the University of Erlangen, Germany, and was the leading enroller in the FAST-FFR trial. Achenbach explained the importance of the clinical findings in this trial; “Guidelines mandate that revascularization decisions are based on the presence of ischemia. However, not every stenosis or invasive angiography, even if perceived as ‘severe,’ causes ischemia. Equally important, lesions that do not appear severely stenotic may cause ischemia at times and may hence benefit from revascularization. Invasive angiography in such cases is incomplete without assessment of ischemia. Invasive FFR is considered the standard of care in these situations but adds cost and a significantly higher level of invasiveness to the angiogram, leading to pronounced under-use in clinical reality. Enrolling many patients in the FAST-FFR trial, my team and I experienced how easily the CathWorks FFRangio System can be added to the angiogram. The published trial results convincingly demonstrate the clinical benefit that may be derived. The CathWorks System may turn out to be disruptive technology that changes the workflow in the cath lab - to the benefit of our patients.”
The FAST-FFR trial was rigorous. The data was measured by 19 on-site cath-lab clinicians blinded to the invasive FFR measurements. Angiograms were acquired by dozens of operators at ten hospitals using all four of the major angiography systems (Siemens, Phillips, Canon, and GE). In addition, a majority of subjects were overweight or obese, and 20 percent had calcified lesions. In the presence of all of these real-world conditions, the FFR-angio accuracy was still very high.
William Fearon, M.D., Professor of Cardiology at Stanford Medical Center in Palo Alto, California, was the principal investigator of the trial and lead author. Fearon pointed out that “The results of the FAST FFR trial demonstrate a very high accuracy and strong correlation between the reference standard, pressure wire-derived FFR and FFRangio. Based on these findings, FFRangio has the potential to substantially increase physiologic coronary lesion assessment in the catheterization laboratory, thereby leading to improved patient outcomes.”
The CathWorks FFRangio System is in development and is not yet FDA-cleared.
Find links to the TCT 2018 Late-breaking Cardiovascular Clinical Trials and Videos
Reference:


 October 24, 2025
October 24, 2025 









