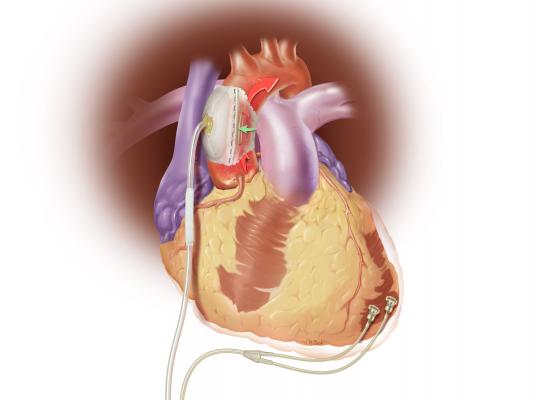
C-Pulse System image courtesy of Sunshine Heart
September 8, 2015 — Sunshine Heart Inc. announced the U.S. Food and Drug Administration (FDA) has approved an amendment to the stopping rule criteria for the company's COUNTER HF pivotal study for its C-Pulse Heart Assist System. The agency has agreed to change this protocol from "all cause" deaths to specifically, mortality associated with device, procedure or therapy.
Sunshine Heart previously announced on March 6, 2015, a temporary enrollment pause in accordance with the study's original "stopping rule." This particular protocol indicated that, in the event more than three of the first twenty subjects pass away for any reason, including non-device related deaths, the company would work with the FDA to establish a plan before resuming enrollment. An independent Clinical Events Committee (CEC) determined that all four of the reported deaths were adjudicated as being non-device related and, on May 26th, the company announced the FDA's approval to resume enrollment in the COUNTER HF study.
Moving forward, the "stopping rule" has been amended such that COUNTER HF will be halted if more than seven of the first twenty implanted subjects pass away during device support within twelve months of implant. Importantly, in order for a study pause to occur, each mortality event will have to be adjudicated as possibly or definitely related to the procedure, therapy or device.
The C-Pulse Heart Assist System, or C-Pulse System — an investigational device in the United States, Canada and countries that do not recognize the CE mark approval — utilizes the scientific principles of intra-aortic balloon counter-pulsation applied in an extra-aortic approach to assist the left ventricle by reducing the workload required to pump blood throughout the body, while increasing blood flow to the coronary arteries. Combined, these potential benefits may help sustain the patient's current condition or, in some cases, reverse the heart failure process, thereby potentially preventing the need for later-stage heart failure devices, such as left ventricular assist devices (LVADs), artificial hearts or transplants. It may also provide relief from the symptoms of Class III and ambulatory Class IV heart failure and improve quality of life and cardiac function. Based on the results from our feasibility study, we also believe that some patients treated with our C-Pulse System may be able to stop using the device due to sustained improvement in their conditions as a result of the therapy.
For more information: www.sunshineheart.com


 February 03, 2026
February 03, 2026 









