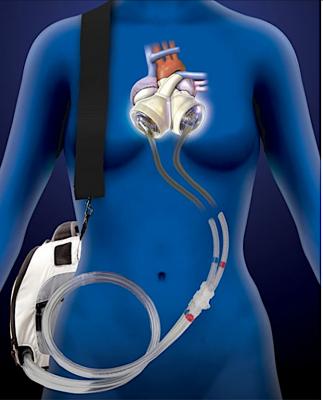
March 10, 2020 — The U.S. Food and Drug Administration (FDA) has cleared the SynCardia Systems 50cc temporary Total Artificial Heart System (50cc TAH-t) as a bridge to transplantation in cardiac transplant eligible patients at risk of imminent death from biventricular failure. With this approval, physicians can now offer the TAH-t to patients with smaller thoracic dimensions including small statured adults, and some pediatric patients. The SynCardia TAH-t is the only dischargeable biventricular support device approved by the FDA which can allow patients to recover at home while maintaining high priority (UNOS Status 2) for a donor heart.
Recent data shows that the prevalence of heart failure (HF) is continuing to rise with an estimated 6.2 million adults ≥20 years of age experiencing HF between 2013 and 2016, up from 5.7 million between 2009 and 2012.[1] For advanced heart failure patients for whom medical therapy alone is no longer an option and both sides of the heart are no longer able to support normal circulation, the TAH-t can serve as a bridge to transplant providing immediate hemodynamic restoration and clinical stabilization, leading to end-organ recovery while awaiting the availability of a donor heart.
“By its very nature the process of allocating a limited number of precious donor hearts to a growing population of patients needing transplantation can lead to some people having to wait longer than we might like,” said Kim Rallis, transplant executive director at the University of Louisville Health – Jewish Hospital and OPTN/UNOS Board Member, “The total artificial heart plays an important role in giving hope to some patients waiting for a heart transplant by not only allowing them to survive to transplant but to thrive after transplant by allowing for end organ recovery.”
Discussing what the 50cc TAH-t means to SynCardia and the community, Don Webber, CEO of SynCardia, said the 50cc TAH-t represents the success of a collaborative effort between the company, its Medical Advisory Board and other clinical leaders in the field of mechanical circulatory support (MCS) offering hope to more patients suffering from end stage biventricular failure. He said both the 50cc and 70cc continue to show excellent clinical results with 77.1 percent of patients in a recent analysis achieving positive outcomes. With the introduction of the 50cc device, he said the device will bring this potentially lifesaving therapy to more patients,[2]
“The approval of the 50cc TAH-t is a significant step forward to providing this unique technology for many children and women who in the past did not have the opportunity because of their size. The TAH-t provides hope for those patients who are not well supported by our traditional forms of mechanical circulatory assistance.” said David Morales, M.D., director of congenital heart surgery at Cincinnati Children’s Hospital.
“It works great in children and small women providing the physiologic support they require in a package their stature can accommodate," said Francisco Arabia, M.D., physician executive of the advanced heart program at Banner University Medical Group.
The 50cc TAH-t System received CE mark in December 2014 and Health Canada approval in January 2016.
SynCardia has also submitted a PMA supplement to FDA for expanding the 70cc TAH-t indication for use to include destination therapy, which would provide this technology to patients who are not eligible for a cardiac transplant. Approval is anticipated later this year.
For more information: https://syncardia.com/clinicians/about-syncardia/
References:
2. Data on File at SynCardia Systems LLC. Tucson, AZ. Patients implant from 10/18/18 to 8/31/19 (n=35). Positive result defined as survival to heart transplant or >180 days on support.

 May 30, 2025
May 30, 2025