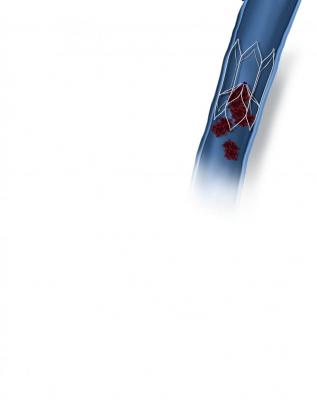
December 4, 2018 — BTG plc announced the first patients outside of a clinical trial have been successfully implanted with the BTG Sentry device, a bioconvertible inferior vena cava (IVC) filter. The BTG Sentry filter is designed to provide protection from pulmonary embolism (PE) for the period of transient risk and then bioconvert to leave a patent, unobstructed IVC lumen. This eliminates the need to retrieve and addresses the typical filter-related complications associated with conventional IVC filters.
Ayad K.M. Agha, M.D., director of interventional radiology and an interventional radiologist at Cardiovascular Interventional Radiology Centers in Phoenix, who performed the procedure on one of his patients, said: “Traditional IVC filters are sometimes associated with a variety of concerns. Placing the BTG Sentry filter gives me confidence in reducing potential complications seen with conventional filters. Using the BTG Sentry IVC filter only requires one visit which means my patient doesn’t have to worry about coming back to make sure the filter is retrieved. This is better for the patient and their families and removes the risk of any complications that may arise on a follow-up procedure”
The BTG Sentry filter is supported by two years of data available through the SENTRY Trial. The data was recently presented on podium at VIVA in Las Vegas, and demonstrated no instances of filter tilt, migration, embolization, fracture or IVC perforation through 24-months of imaging-intensive follow-up.
U.S. Food and Drug Administration 510(k) clearance for the Sentry device was obtained in 2017.
For more information: www.btgplc.com


 November 14, 2025
November 14, 2025 









