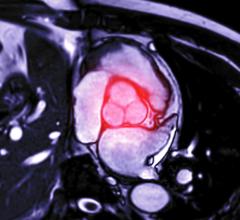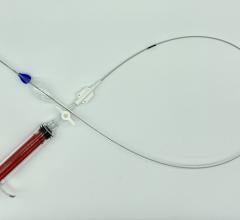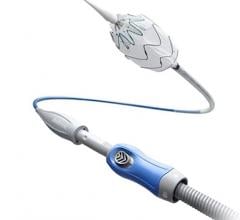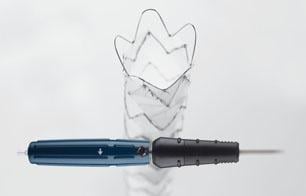
January 28, 2016 — In November, following the U.S. Food and Drug Administration approval of Cook Medical’s Zenith Alpha Thoracic Endovascular Graft, the first patient was treated with the device in the United States.
Zenith Alpha Thoracic is indicated for the endovascular treatment of patients with isolated lesions of the descending thoracic aorta (not including dissections) having vascular anatomy suitable for endovascular repair. With a 16-20 French delivery system, the device was developed to provide vascular access and delivery with a lower profile device which allows physicians to consider thoracic endovascular aortic repair (TEVAR) options for patients who otherwise may not have been candidates for larger-profile devices.
The Cleveland Clinic was the first to treat a patient with the device in the U.S. following the approval.
For more information: www.cookmedical.com


 September 18, 2025
September 18, 2025