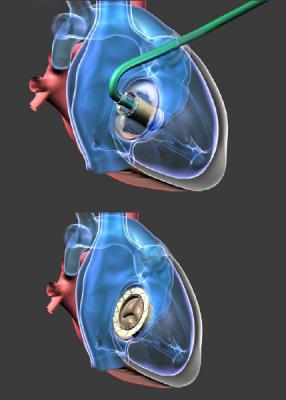
February 22, 2018 — NaviGate Cardiac Structures Inc. (NCSI)announced that on Feb. 2, 2018, its catheter-guided Gate valved-stent bioprosthesis became the first Canadian orthotopic valve replacement to treat severe tricuspid regurgitation. The procedure was performed at the Quebec Heart and Lung Institute, Laval University (Quebec City, Canada) by the Institute’s Cardiac Team, led by Josep Rodés-Cabau, M.D., interventional cardiologist, an internationally known expert in tricuspid disease therapy; and François Dagenais, M.D., chief of cardiac surgery at the institute. They performed the Health Canada-sanctioned Expanded Access (Compassionate Use) procedure in the patient who presented with severe symptomatic tricuspid regurgitation.
The patient, a 79-year old male, was deemed at high-risk for standard cardiac surgery and unable to withstand cardiopulmonary external circulation. Rodés-Cabau, who has performed multiple procedures for atrioventricular valve dysfunction using various repair devices, said: “The NaviGate bioprosthesis is the only transcatheter device in development that has been used to completely replace the native tricuspid valve and restore its function in the human.” Rodés-Cabau proposed the NCSI Gate as the only viable treatment option for this patient. The replacement procedure, performed in less than 30 minutes after the patient was prepared, minimized fluoroscopic exposure to both patient and operator, as well as contrast imaging media quantity known to be deleterious to the patient’s kidney.
There were no immediate complications and an excellent result was obtained, which allowed the patient to be discharged within a few days after a satisfactory recovery from the procedure. The patient said that for a long time before this procedure, he had experienced loss of balance and generalized weakness. Now, with feelings of relief a few days after the procedure, he felt much better and all was returning to normal.
In three of 10 previously treated patients, the Gate bioprostheses were implanted through the jugular vein of the neck through a tiny puncture. “Because the patient’s anatomy did not allow jugular access to the tricuspid valve,” Dagenais explained, “we chose an alternate path. Fortunately, with a small incision between a pair of right ribs, a direct path for the catheter led to quick implantation of the replacement valve. We must understand that the technology will evolve and soon the great majority of patients will receive their replacement valve through the neck veins,” he concluded.
Élisabeth Bédard, M.D., the cardiologist aiding the navigation through imaging, explained: “We see these patients continuously, showing edema of their lower limbs, shortness of breath and their chests full of liquid. We give them medications to relieve them of some fluid but in a short time come to an end where we cannot help them anymore. Suddenly, we see a superb new technology that may help our patients.”
The patient now joins a cohort of 10 patients, seven with valves functioning over 5 months, and he joins in two weeks a cohort of three patients with well-functioning Gate bioprostheses that has reached greater than 30 days.
The valve replacement system is developed at NaviGate Cardiac Structures Inc. and includes novel technology on tissue dehydration that extracts toxic fixative glutaraldehyde. That technology licensed from Cleveland Clinic Foundation’s original research and was co-licensed to Edwards Lifesciences from NCSI for fees and royalties. It is believed that such technology may have potential to increase the longevity of the tissue valvular mechanism.
The Gate atrioventricular valved stent is not approved for use in the U.S. The patient implants described in this news release were performed under compassionate pleas sanctioned by Health Canada, European Health agencies and the U.S. Food and Drug Administration (FDA).
For more information: www.navigatecsi.com


 December 24, 2025
December 24, 2025 









