April 8, 2024 — Implantation of the Impella CP micro-axial flow pump in the hours after a heart attack significantly increased the rate of survival at six months among people suffering cardiogenic shock, according to a study presented at the American College of Cardiology’s Annual Scientific Session.
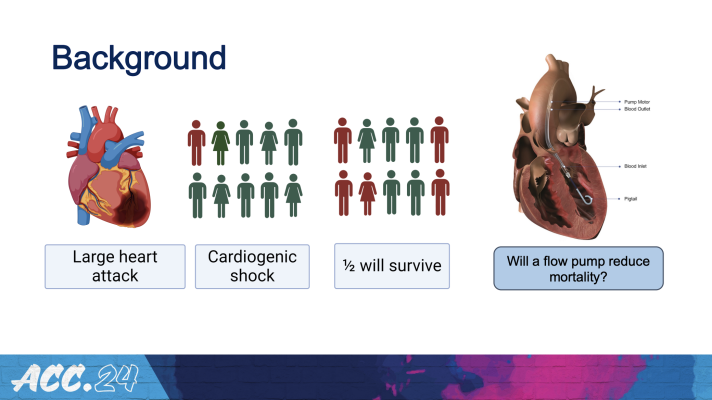
Cardiogenic shock occurs in about 5% to 10% of heart attacks and is among the main drivers of heart attack-related deaths. It occurs when the heart suddenly cannot pump enough blood to meet the body’s needs, depriving the heart and other vital organs of oxygen and often leading to death without immediate treatment. The study, which met its primary endpoint, suggests that by helping to restore the flow of oxygen-rich blood to the body, the device can help to improve survival in these severe cases.

Jacob E. Møller, MD
“This is the first time in a very long time that we have a positive study for managing cardiogenic shock,” said Jacob E. Møller, MD, professor in the Department of Cardiology at the Odense University Hospital in Denmark, consultant at the cardiac intensive care unit of Copenhagen University Hospital Rigshospitalet and the study’s lead author. “I think this will be a routine device that will be used in these desperately ill patients.”
The Impella CP is a small percutaneous pump placed within the heart’s left chamber, where it expels oxygenated blood from the left ventricle to the body with a flow rate of up to 3.5 liters per minute. Previous trials evaluating the potential benefits of this and other heart pumps for cardiogenic shock have had mixed results. The new trial, called DanGer Shock, is the first trial powered to examine whether the use of micro-axial flow pumps can improve survival in ST-elevation myocardial infarctions (STEMI, the most serious type of heart attack) that are complicated by cardiogenic shock.
The trial enrolled 360 patients treated for STEMI with cardiogenic shock at 14 centers in Denmark, Germany and the United Kingdom. Patients who suffered out-of-hospital cardiac arrest with coma and increased risk of brain damage were excluded from the trial. Researchers randomly assigned patients to receive standard care or standard care plus treatment with an Impella CP pump. Participants were randomized before, during or up to 12 hours after receiving treatment in the cardiac catheterization laboratory, depending on when cardiogenic shock was diagnosed.
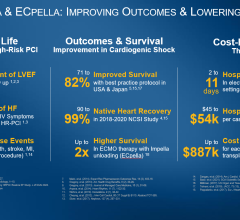
Among 355 patients who were included in the analysis, 58.5% of those who received standard care alone and 45.8% of those who received the Impella pump had died at six months after randomization; there was a 13 percentage point absolute reduction in the rate of death, the study’s primary endpoint, in favor of the heart pump. In addition, the results showed a reduction in a composite endpoint of additional mechanical heart support, heart transplant or death among patients who received the heart pump, but there was no difference between the two groups in the number of days out of the hospital.
“What was a surprise for us was that the benefit seems to persist beyond 30 days,” Møller said. “It’s not only that we are saving lives, it looks like we are also saving myocardium [heart muscle] so the patients keep surviving, and the survival curves continue to separate beyond the first 30 days.”
However, the results also showed significantly higher rates of complications among patients who received the heart pump, including bleeding, limb ischemia, renal replacement therapy and sepsis.
“It doesn’t come without a cost—we see significantly more serious complications in the Impella treated patients,” Møller said. “Overall, we have more complications, but we also save lives.”
Møller said that the study is not generalizable to all cases of cardiogenic shock as the trial was more selective than previous trials in identifying patients who were most likely to be able to benefit from the use of a heart pump, for example, by excluding those with a risk of brain damage. However, within this patient population, he said the results are likely translatable beyond northern Europe to large centers with the necessary expertise to employ the device.
Subgroup analyses suggested that patients with very low blood pressure and those with lesions in more than one coronary artery may see a greater benefit from the Impella pump. Møller said that further studies are needed to assess the benefits in more diverse patient populations and to examine how the duration of mechanical support might affect the rate of severe complications and identify opportunities to further optimize practices to minimize complications.
The study was funded by the Danish Heart Foundation and Abiomed/Johnson & Johnson, maker of the Impella CP.
This study was simultaneously published online in the New England Journal of Medicine at the time of presentation.
For more information: www.acc.org


 April 28, 2023
April 28, 2023