August 25, 2022 — Imperative Care, Inc. announced that new data evaluating the utility of the Zoom Stroke Solution were presented at the World Federation of Interventional and Therapeutic Neuroradiology (WFITN) 2022 Annual Meeting in Kyoto.
Results from an independent single-center, multi-operator experience evaluating the use of the Zoom Stroke Solution for the treatment of patients with middle cerebral artery (MCA) M2 occlusions were presented by Collin Torok, M.D., Neurointerventional surgeon at Midwest Radiology in Minneapolis-St. Paul.
“More and more physicians are treating distal vessel occlusions despite their location in the brain — which can be more challenging to reach than other types of occlusions — resulting in a meaningful clinical impact. We are pleased to see the results from this single-center experience demonstrating the unique capabilities of the Zoom Reperfusion Catheters for the treatment of MCA M2 segment occlusions,” said Daniel Davis, President and COO of Imperative Care.
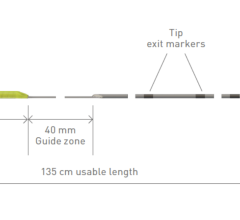
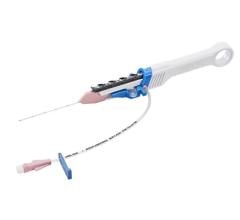
“The Zoom Stroke Solution is the only system that pairs an intracranial access catheter, Zoom 88, with a range of purposefully designed vessel-matching reperfusion catheters, Zoom 35, 45, 55 and 71, and pump that can help reach these types of occlusions and enable fast and effective clot ingestion. We look forward to continued studies to evaluate mechanical thrombectomy in these vessels and further emphasize the clinical efficacy of our technology,” said Davis.
Endovascular approaches for distal occlusions can be more challenging and are associated with a higher risk of periprocedural complications as a result of a longer path to the occlusion and a smaller vessel diameter.1 This study was undertaken to assess the safety and reperfusion success of aspiration thrombectomy in patients with these occlusions.
Results from the study showed that TICI 2b/3 reperfusion was achieved in 92.7% (38/41) of patients and TICI 2c/3 reperfusion was achieved in 63.4% (26/41) of patients. The median score on the National Institute of Health Stroke Scale decreased significantly from 10.5 (IQR 7.0 to 14.5) at arrival to 1.5 (0 to 6.0) at discharge, p < 0.001. Median time from access to first aspiration attempt was 10 (6.6 to 16.5) minutes and median time from access to final reperfusion was 21.5 (16.0 to 31.8) minutes. There was one instance (2.4%) of symptomatic intracranial hemorrhage in this study cohort. There were no perforations or other reperfusion catheter related complications.
"Distal vessel occlusions are considered the next frontier in mechanical thrombectomy for stroke treatment. The data from this study offer additional clinical evidence that aspiration thrombectomy using the Zoom Stroke Solution is both safe and effective for occlusions in the MCA M2 segment. Furthermore, Zoom 88 provides an excellent intracranial access catheter platform for distal occlusions. Even in the most tortuous cases, we have more than halved our procedural times with rapid initial and repeat passes to the distal circulation as needed,” said Dr. Torok, lead author of the study.
For more information: https://imperativecare.com
References:
+ Dr. Torok is a paid consultant for Imperative Care.
- Coutinho JM, Liebeskind DS, Slater LA, et al. Mechanical thrombectomy for isolated M2 occlusions: a post hoc analysis of the STAR, SWIFT, and SWIFT PRIME studies. AJNR Am J Neuroradiol 2016; 37: 667–672


 January 29, 2026
January 29, 2026