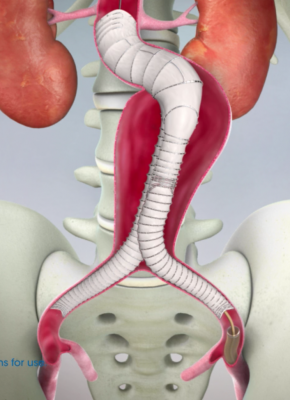
January 11, 2017 — Lombard Medical Inc. recently announced approval from the Japanese Ministry of Health, Labour and Welfare for its IntelliFlex Low Profile (LP) Delivery System for the Aorfix Endovascular Stent Graft. Commercial launch will follow reimbursement approval, which is anticipated in February 2017.
This new delivery system is used in conjunction with the Aorfix endovascular stent graft, the only abdominal aortic aneurysm (AAA) stent graft with global regulatory approval to treat aortic neck angulation up to 90 degrees, according to Lombard Medical. The IntelliFlex LP Delivery System features a low profile, compact and ergonomic design that provides intuitive control of Aorfix during deployment, even in challenging anatomy. The system features an integrated exchange sheath allowing ancillary devices to be introduced during the endovascular AAA procedure, avoiding the need for sheath exchanges and thus potentially reducing blood loss, vessel trauma, procedure cost and time.
The endovascular aneurysm repair (EVAR) market in Japan is estimated at 10 percent of the global market and has been growing at an average rate of approximately 18 percent. In Japan, there are approximately 400 physicians at 200 clinics performing EVAR, and it is estimated that approximately 55 percent of Japanese AAA patients are treated using this method.
For more information: www.lombardmedical.com


 September 18, 2025
September 18, 2025 









