Andreas Brieke, M.D., director of mechanical circulatory support, heart failure physician and site principal ...
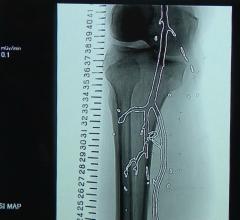
Cardiva Medical Inc. announced the company has received premarket approval (PMA) from the U.S. Food and Drug Administration (FDA) for the Vascade MVP Venous Vascular Closure System. Vascade MVP is the first and only vascular closure system, according to Cardiva, designed and labeled specifically for multi-site venous closure – for 6-12 French inner diameter sheaths. This access site approach and size range is the standard in electrophysiology procedures such as cardiac ablation and left atrial appendage closure.
December 20, 2018 — The U.S. Food and Drug Administration (FDA) has warned Genetech Inc. and its president, Edwin N ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
A new set of appropriate use criteria (AUC) released Dec. 17 by a group of cardiovascular professional societies provide guidelines for peripheral artery interventions. The purpose of the AUC is to provide guidance to clinicians who may refer patients for revascularization treatments and to interventionalists and surgeons themselves. With the field of peripheral artery disease constantly evolving, it is imperative to offer tools and resources that physicians can utilize to provide the best care for their patients.
As part of its plans to spin off its healthcare division into a separate company, GE reportedly submitted the paperwork Dec. 19 for an initial public offering (IPO) of the standalone company. Inside sources familiar with the situation told Bloomberg that the public filing will likely happen in spring 2019. Numerous sources reported that the news caused a significant jump in the parent company’s stock price.
Irish medical device company AuriGen Medical won the prestigious Global Innovation Award at the International Conference for Innovations in Cardiovascular Systems (ICI), Dec. 2-4 in Tel Aviv, Israel. AuriGen Medical, which specializes in the treatment of atrial fibrillation, is developing the first heart implant to treat both the stroke and heart failure risk associated with this often fatal condition.The Global Innovation award attracted entries from the best medical device and pharmaceutical start-ups from around the world.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
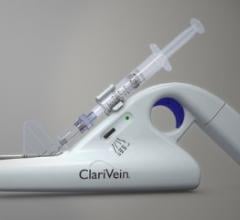
Merit Medical Systems Inc. announced that it has acquired substantially all of the assets of Vascular Insights LLC, based in Quincy, Mass. Vascular Insights’ primary assets are the ClariVein IC and ClariVein OC specialty infusion and occlusion catheter systems, which have been utilized in more than 120,000 cases to treat superficial venous disease, particularly below the knee (BTK), and venous leg ulcers (VLU). The ClariVein systems address a $700 million global market. The ClariVein IC system has 510(k) clearance from the U.S. Food and Drug Administration (FDA), the ClariVein OC system is CE-marked, and the systems are covered by 43 patents issued worldwide.
A judge of the Federal District Court for the Northern District of Texas ruled the Affordable Care Act (ACA) unconstitutional in a shocking decision that could impact the health insurance coverage of millions of Americans. A group of Democratic state attorneys general plans to bring the case to the 5th Circuit U.S. Court of Appeals, and the Trump administration said the law will remain in place until the appeal process is complete.
Among otherwise healthy people, a daily dose of aspirin does not save lives and causes additional bleeding, a new analysis has found. The meta-analysis of 11 aspirin therapy clinical trials involving more than 157,000 healthy individuals since the 1980s found the drug does not reduce deaths, heart attacks and strokes. Low-dose aspirin users were also about 50 percent more likely to have major bleeding compared with those who did not use aspirin, the researchers concluded. The findings were published Dec. 17 in the European Heart Journal.
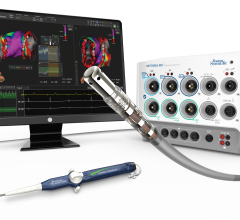
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Shockwave Medical announced a new investment and collaboration agreement with Abiomed Inc. As outlined by the agreement, Abiomed will invest $15 million in Shockwave and the two companies will collaborate on a training and education program in the United States and Germany focused on the benefits of complementary use of their respective technologies.
Digital health company Analytics 4 Life and Actelion Pharmaceuticals Ltd. announced a collaborative agreement to investigate the use of Analytics 4 Life’s diagnostic imaging technology in pulmonary hypertension. The first 500-person clinical study has been initiated for this serious, life-threatening condition affecting millions of people worldwide.
The Department of Health and Human Services (HHS) recently entered into an interagency agreement with the National Aeronautics and Space Administration (NASA) to coordinate scientific research efforts on healthcare. Eric D. Hargan, deputy HHS secretary, wrote the following column about the implications of the agreement.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
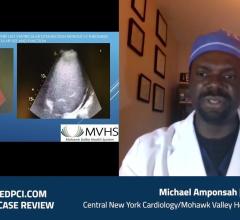
Michael Amponsah, M.D., FACC, an interventional cardiologist at Mohawk Valley Health System, shares a case of Impella CP ...
December 12, 2018 — In stroke, time saved on imaging is time gained in the treatment window. The recently updated ...

A jury in the U.S. District Court for the District of Delaware determined Dec. 11 that the Boston Scientific U.S. patent 8,992,608 is valid and that Edwards Lifesciences' Sapien 3 Aortic Valve infringes this patent. The court ruled that Edwards owes Boston Scientific $35 million in infringement damages through the end of 2016. Additional damages and interest incurred from 2017-2018 will be determined by the court in post-trial motions.

 December 20, 2018
December 20, 2018