Population health enablement company Higi announced their commitment to implement the 2017 American College of Cardiology (ACC)/American Heart Association (AHA) blood pressure guidelines across the 11,000 U.S. Food and Drug Administration (FDA)-cleared Higi stations nationwide. Higi said it will use its stations at retail stores and community locations to engage consumers in their health, helping them understand their biometric measurements and connecting them with the appropriate care for earlier intervention and better management of hypertension treatment plans.
Physicians, trainees and even laypeople can now stand right beside an expert radiologist as he performs one of the most difficult medical procedures of its kind – in virtual reality
Roxana Mehran, M.D., FACC, FACP, FCCP, FESC, FAHA, FSCAI, professor of medicine and director of interventional ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
A discussion with Ron Waksman, M.D., associate director of the division of cardiology and director of cardiovascular ...
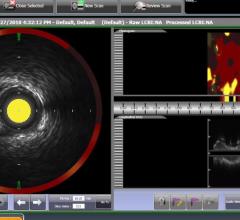
March 27, 2018 — Edwards Lifesciences Corp. announced that enrollment is complete in the computed tomography (CT) ...

There were several notable presentations of new data on cardiovascular technologies at the recent 2018 American College ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
March 27, 2018 — Depression may increase the risk for atrial fibrillation, the most common heart rhythm disorder that ...
March 26, 2018 — Measurement of cardiac troponin in stable patients with chest pain using the ultra-sensitive Singulex ...
James Januzzi, M.D., Roman W. DeSanctis Endowed Distinguished Clinical Scholar in Medicine, and director of the Dennis ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Materialise NV became the first company in the world to receive U.S. Food and Drug Administration (FDA) clearance for software intended for 3-D printing anatomical models for diagnostic use. The company said leading hospitals are adopting integrated 3-D printing services as part of their medical practices as they recognize the added value it brings to personalized patient care.
David Lanfear M.D., FACC, head of advanced heart failure and cardiac transplantation, Henry Ford Hospital, Detroit ...
Patients with foot ulcers or gangrene who received the experimental drug JVS-100 did not show evidence of faster wound healing, compared with those receiving a placebo, in a study presented at the American College of Cardiology’s 67th Annual Scientific Session, March 10-12 in Orlando, Fla.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
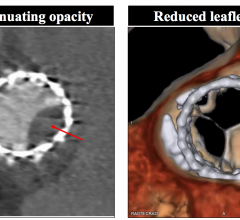
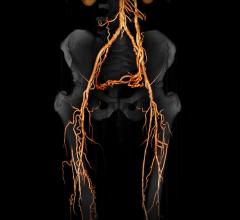
March 23, 2018 — A relatively inexpensive 3-D-printed model of a patient's blood vessels is as effective as current ...
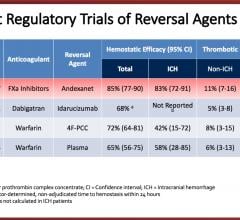
March 22, 2018 — The experimental drug andexanet was associated with control of serious bleeding in patients taking a ...
Cardiovascular Systems Inc. (CSI) recently announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the OrbusNeich 1mm Sapphire II Pro coronary balloon.


 March 28, 2018
March 28, 2018