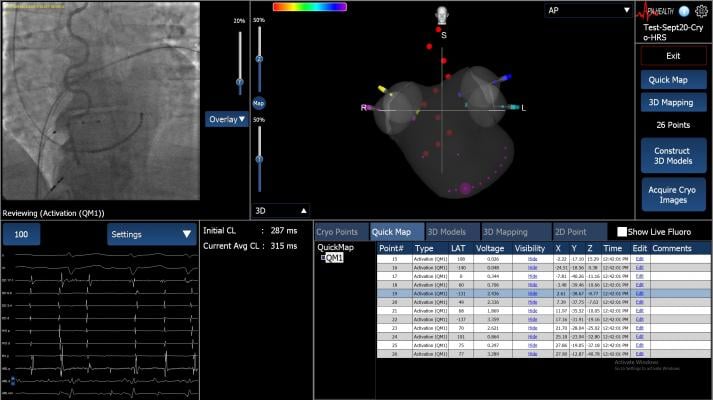
May 3, 2018 — APN Health LLC announced its Navik 3D mapping system is commercially released and in clinical use. The first clinical case using the technology was performed on March 22 at St. Luke’s Medical Center in Milwaukee.
Navik 3D is intended for catheter-based atrial and ventricular mapping when treating cardiac arrhythmias using compatible catheters and acquired data from compatible fluoroscopy systems and patient recording and monitoring systems. The system is intended to provide 3-D location of these catheters from acquired 2-D fluoroscopic images. The device allows real-time display of cardiac maps in a number of different formats, including anatomical maps, cardiac electrical activation maps and cardiac voltage maps.
Navik 3D is the first open platform cardiac mapping technology that does not require any custom equipment, according to the company. It can identify 3-D catheter locations in the heart and create three-dimensional maps of the cardiac chamber of interest.
The system is being rolled out to select U.S. and international customers, with a full roll-out expected n the next 6 to 12 months.
For more information: www.apnhealth.com


 January 29, 2026
January 29, 2026 









